
To compare the efficacy of the traditional Chinese medicine SanJieZhenTong (SJZT) capsules versus gonadotropin-releasing hormone analogs (GnRHa) or oral contraceptives (OCs) in the postoperative treatment of moderate-to-severe endometriosis.
In this prospective clinical trial, women with stage III-IV endometriosis according to the revised American Fertility Society scoring system received three doses of GnRHa immediately after laparoscopic conservative surgery, followed by random assignment to receive treatment with SJZT, GnRHa, or OCs for another 6 months. The primary endpoint was 2-year recurrence, and the secondary endpoints were adverse events, changes in physical function, and quality of life (QoL). Recurrence was assessed using Kaplan-Meier curves and log-rank tests. Generalized estimating equations were used to determine the parameters of the secondary endpoints.
A total of 66 women were randomly assigned to the SJZT (n = 21), GnRHa (n = 21), and OCs (n = 24) groups. At a median follow-up of 22 months, no difference in recurrence was found (P = 0.72), with one (4.8%), two (9.5%), and one (4.2%) incidence in the SJZT, GnRHa, and OCs groups, respectively. Expectedly, the incidence of side effects such as hot flush, insomnia, and arthralgia in the SJZT and OCs groups was significantly lower than that in the GnRHa group (P = 0.00). In addition, the female sexual function index was significantly improved in the SJZT group, with a higher value than that in the GnRHa (odds ratio [OR] = 5.25, 95% confidence interval [CI]: 2.09-13.14, P = 0.00) and OCs (OR = 3.94, 95% CI: 1.58-9.83, P = 0.00) groups.
SJZT showed more effective pain relief and QoL improvement in patients with moderate-to-severe endometriosis than GnRHa or OCs did. Fewer adverse events than those observed with other agents indicate that this alternative medicine, SJZT, could be a novel option for the long-term management of endometriosis.

This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
For reprints contact: WKHLRPMedknow_reprints@wolterskluwer.com
© 2021 Reproductive and Developmental Medicine | Published by Wolters Kluwer-Medknow
Endometriosis refers to the presence of active endometrial tissue, including the glands and stroma, outside the endometrium. The prevalence of endometriosis in women of reproductive age is up to 10%-15%[1] and 50% in infertile patients.[2] Moreover, new cases reported globally show an increasing trend, and as of 2017, approximately 176 million women worldwide have been affected by this disease.[3] Endometriosis, an estrogen-dependent disease with a high recurrence rate, has been recognized as the most common and most difficult gynecological chronic disease, leading to significant negative effects on the quality of life (QoL) and work efficiency of women because of symptoms such as infertility, progressive chronic pelvic pain, severe dysmenorrhea, pain during defecation, and dyspareunia.[4,5]
Because endometriosis patients are relatively young, most choose conservative surgery, which preserves the fertility function; however, subsequent recurrence after conservative surgery is a challenge for endometriosis treatment. Currently, there is no consensus on an effective treatment for short-term control of recurrence. At present, global drug treatment of endometriosis costs as much as 1.2 billion US dollars per year,[6] which has placed a huge economic burden on the society.
Gonadotropin-releasing hormone analogs (GnRHa) and oral contraceptives (OCs) are common and effective hormonal drugs for endometriosis treatment.[7] A large randomized, multicenter trial demonstrated that 3 and 6 months of GnRHa treatment resulted in pain relief and a recurrence rate that were not significantly different.[8] However, whether the extended use of GnRHa, such as for 9 months, would be more beneficial is unknown. Similarly, OCs are also one of the first-line drugs for the long-term management of endometriosis, but the benefit of OCs compared to that of GnRHa is still unclear.
SanJieZhenTong (SJZT) capsule is a traditional Chinese medicine formulation; as an alternative medicine, SJZT has shown beneficial effects in the management of endometriosis. However, because of poor design and implementation, it is difficult to objectively determine whether this traditional Chinese medicine formulation has a curative effect in treating endometriosis.[9,10] Therefore, the therapeutic effect of SJZT on endometriosis needs to be further explored. In this study, we performed a prospective, randomized controlled clinical trial to compare the effects of SJZT, GnRHa, and OCs on recurrence, symptom control, QoL, and organ function in the long-term management of moderate and severe endometriosis.
This was a registered (ChiCTR-IPR-17013692), prospective, three-arm, randomized controlled trial (RCT) conducted with the approval of the Institutional Review Board Ethics Committee of the Obstetrics and Gynecology Hospital of Fudan University (2017-50). The randomization number was generated using the STATA statistical software package by Dr. Q.T., who did not participate in other study procedures. The patients received three 28-day cycles of GnRHa (3.75 mg) postoperatively, and at the fourth cycle, each enrolled patient was numbered sequentially and assigned a random number using the random number table. Next, according to random numbers, Dr. Q.T. divided the patients into the following three groups that were treated as indicated: SJZT (1.6 g, three times a day [t.i.d.]), GnRHa (3.75 mg, intramuscularly [i.m.] every 28 days [q28d]), and OCs (drospirenone 3 mg and ethinyl estradiol 0.02 mg, daily [q.d.]), for 6 consecutive months. "Add-back" therapy (estradiol valerate 0.5 mg/d plus dydrogesterone 5 mg/d)[11] was prescribed for patients in the GnRHa group with estradiol levels <30 pg/mL.
The severity of endometriosis was evaluated according to the revised American Fertility Society (rAFS) scoring system and double-checked by two doctors independently (Dr. Y.Z. and Dr. J.R.). Patients diagnosed with moderate-to-severe endometriosis (rAFS score >15, stage III-IV) after conservative surgery (cystectomy, or excision of other endometriotic lesions, or both) were prospectively recruited. Eligible patients were >18 and <45 years of age and had no fertility planning in the 1st year postoperatively. Patients were also expected to be mentally and physically capable of expressing their complaints and answering questions. Before enrollment, all patients signed an informed consent form.
The following patients were excluded: patients (1) with gynecological malignant tumors, genital tuberculous nodules, or endometriosis undergoing total hysterectomy with bilateral adnexal resection; (2) with internal diseases involving the cardiovascular, neural, digestive, urinary, or hematopoietic systems; (3) with other gynecological endocrine diseases such as polycystic ovary syndrome; (4) who were pregnant or lactating; (5) who were allergic to the experimental drugs (SJZT, GnRHa, or OCs); (6) who were participating in other clinical trials; (7) with hemoglobin levels <80 g/L; (8) who were diagnosed with rAFS stage I-II; and (9) with a family history of thrombosis or thrombotic disease.
Patients were asked to undergo checkups preoperatively and 1, 3, 6, 12, and 24 months postoperatively, which were recorded as preoperative or postoperative V1, V3, V6, V12, and V24, respectively. The workup included medical history, physical examination, and ultrasound findings. The visual analog scale (VAS) was used to assess endometriosis-related pain and discomfort. The modified short form 36 (SF36) questionnaire was used to evaluate the health-related QoL including body pain, physical functioning, role-physical, general health, vitality, social functioning (SF), role-emotional, mental health, and reported health transition. The Urogenital Distress Inventory-6 (UDI-6) was used to evaluate the urinary function, the Colorectal-Anal Distress Inventory-8 (CRADI-8) for digestive function, the female sexual function index (FSFI) for sexual satisfaction, and the Kupperman Index (KI) for menopausal symptoms. Any side effects related to SJZT, GnRHa, or OCs were recorded.
The primary endpoint of 2-year recurrence was expressed as recurrence-free survival, which is defined as the time from primary surgery to disease recurrence. Recurrence was defined as:[12] (1) symptomatic recurrence: VAS >5 in women who had pain symptoms preoperatively after the primary treatment; (2) clinical findings indicative of recurrence: pelvic tenderness, pelvic masses, or nodulations found by pelvic examination; and (3) ultrasonic/radiological diagnosis of recurrence: newly found pelvic mass that matches the diagnosis of endometriosis via ultrasound/magnetic resonance imaging. The secondary endpoints were the (1) efficacy of symptom control (VAS scores) and (2) change in QoL, menopausal symptoms, sexual function, and the digestive and urinary system.
Statistical analyses were performed using IBM Statistical Package for the Social Sciences (SPSS) software for Windows, version 23.0 (IBM, Armonk, NY, USA). Continuous variables for the demographic and surgical information were compared using a one-way analysis of variance with Bonferroni’s correction. Fisher’s exact test was used to analyze categorical characteristics and incidences of any adverse events. Because no patients changed treatment groups midway, the efficacy and safety were assessed in the intention-to-treat population, which included all the patients treated for >1 month after randomization. Kaplan-Meier curves were compared using log-rank tests. In addition, generalized estimating equations were used to determine the statistical significance of differences in efficacy among the three therapies with respect to the secondary endpoints with repeated measures over time. Statistical significance was set at P < 0.05.
In December 2017, 66 eligible patients among approximately 654 patients with endometriosis who underwent operations performed by teams directed by Dr. X.Y. and Dr. Z.Z. (from November 2017 to March 2019) in the Obstetrics and Gynecology Hospital of Fudan University were enrolled and randomly assigned to the SJZT, GnRHa, and OCs groups [Figure 1]. Demographic and baseline characteristics and detailed treatment information are provided in Table 1.

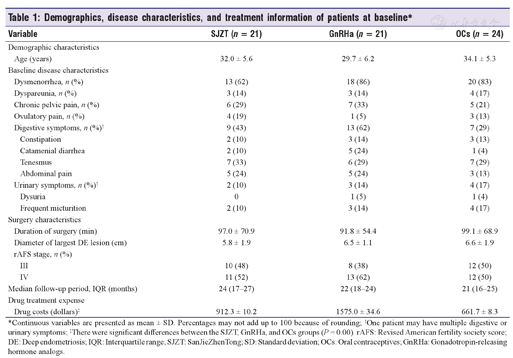
Demographics, disease characteristics, and treatment information of patients at baseline*
Demographics, disease characteristics, and treatment information of patients at baseline*
| Variable | SJZT (n = 21) | GnRHa (n = 21) | OCs (n = 24) | ||
|---|---|---|---|---|---|
| Demographic characteristics | |||||
| Age (years) | 32.0 ± 5.6 | 29.7 ± 6.2 | 34.1 ± 5.3 | ||
| Baseline disease characteristics | |||||
| Dysmenorrhea, n (%) | 13 (62) | 18 (86) | 20 (83) | ||
| Dyspareunia, n (%) | 3 (14) | 3 (14) | 4 (17) | ||
| Chronic pelvic pain, n (%) | 6 (29) | 7 (33) | 5 (21) | ||
| Ovulatory pain, n (%) | 4 (19) | 1 (5) | 3 (13) | ||
| Digestive symptoms, n (%)† | 9 (43) | 13 (62) | 7 (29) | ||
| Constipation | 2 (10) | 3 (14) | 3 (13) | ||
| Catamenial diarrhea | 2 (10) | 5 (24) | 1 (4) | ||
| Tenesmus | 7 (33) | 6 (29) | 7 (29) | ||
| Abdominal pain | 5 (24) | 5 (24) | 3 (13) | ||
| Urinary symptoms, n (%)† | 2 (10) | 3 (14) | 4 (17) | ||
| Dysuria | 0 | 1 (5) | 1 (4) | ||
| Frequent micturition | 2 (10) | 3 (14) | 4 (17) | ||
| Surgery characteristics | |||||
| Duration of surgery (min) | 97.0 ± 70.9 | 91.8 ± 54.4 | 99.1 ± 68.9 | ||
| Diameter of largest DE lesion (cm) | 5.8 ± 1.9 | 6.5 ± 1.1 | 6.6 ± 1.9 | ||
| rAFS stage, n (%) | |||||
| III | 10 (48) | 8 (38) | 12 (50) | ||
| IV | 11 (52) | 13 (62) | 12 (50) | ||
| Median follow-up period, IQR (months) | 24 (17-27) | 22 (18-24) | 21 (16-25) | ||
| Drug treatment expense | |||||
| Drug costs (dollars)‡ | 912.3 ± 10.2 | 1575.0 ± 34.6 | 661.7 ± 8.3 | ||
*Continuous variables are presented as mean ± SD. Percentages may not add up to 100 because of rounding; †One patient may have multiple digestive or urinary symptoms; ‡There were significant differences between the SJZT, GnRHa, and OCs groups (P = 0.00). rAFS: Revised American fertility society score; DE: Deep endometriosis; IQR: Interquartile range; SJZT: SanJieZhenTong; SD: Standard deviation; OCs: Oral contraceptives; GnRHa: Gonadotropin-releasing hormone analogs.
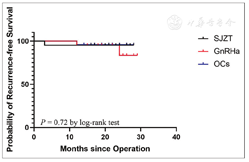
After a median follow-up period of 22 months, four events of symptomatic recurrence were observed in 66 (6.1%) patients without any signs of recurrence on physical examination and imaging checkups. These four patients included one in the SJZT (1/21, 4.8%), two in the GnRHa (2/21, 9.5%), and one in the OCs (1/24, 4.2%) groups. However, there was no significant difference among the three groups, as estimated using Kaplan-Meier analysis [P = 0.72, Figure 2]. The symptoms of recurrence included chronic pelvic pain (VAS = 5) in one patient in the SJZT group, dysmenorrhea (VAS = 8) in two patients in the GnRHa group, and dysmenorrhea (VAS = 7) in one patient in the OCs group.

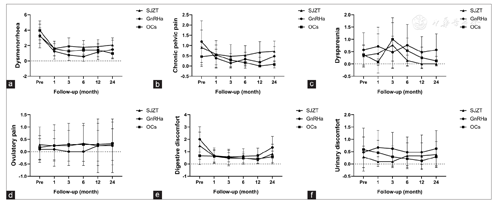
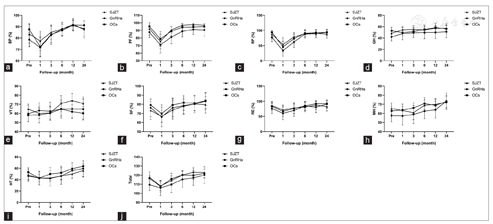
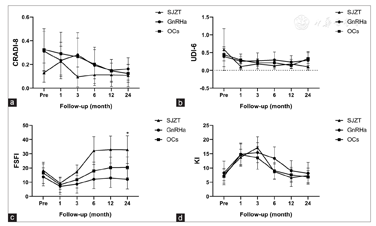
All three regimens showed efficacious pain control. During the 24-month follow-up period, there were no significant differences among the three groups in VAS scores [Figure 3]. In the SJZT, GnRHa, and OCs groups, 62% (13/21), 86% (18/21), and 83% (20/24) of patients had dysmenorrhea; 14% (3/21), 14% (3/21), and 17% (4/24) had dyspareunia; 43% (9/21), 62% (13/21), and 29% (7/24) had digestive symptoms; and 10% (2/21), 14% (3/21), and 17% (4/24) had urinary symptoms preoperatively, respectively [Table 1]. All these symptoms were significantly relieved after treatment. Unfortunately, two patients developed new symptoms after surgery without relief, including one each from the SJZT and GnRHa groups with dysmenorrhea (VAS = 7) and dyspareunia (VAS = 6), respectively. The SF36, CRADI-8, and UDI-6 values were not different among the three groups [Figure 4 and Figure 5a and Figure 3b]. Interestingly, the FSFI scores were significantly improved in the SJZT group compared to those in the GnRHa group (P = 0.00, odds ratio [OR] = 5.25, 95% confidence interval [CI]: 2.09-13.14) and OCs (P = 0.00, OR = 3.94, 95% CI: 1.58-9.83), showing no significant difference between the GnRHa and OCs groups [Figure 5c].



The mean drug costs of the SJZT, GnRHa, and OCs groups were $912.3, $1575.0, and $661.7, respectively (P = 0.00). Furthermore, the drug cost of the GnRHa group included the mean cost of "Add-back" therapy, which was $48.1.
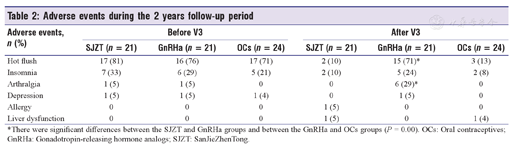
The incidence and types of adverse events differed among the three groups during the different treatment stages [Table 2]. GnRHa decreased the level of estradiol in patients [Figure 6a and Figure 6b], leading to menopausal symptoms. Furthermore, administration of the "add-back" therapy did not relieve the hot flushes and arthralgia in patients in the GnRHa group who still experienced a higher incidence than patients in the SJZT and OCs groups (P = 0.00). However, there was no difference in KI scores among the three groups over time [Figure 5d], and GnRHa treatment increased the incidence of depression.

Adverse events during the 2 years follow-up period
Adverse events during the 2 years follow-up period
| Adverse events, n (%) | Before V3 | After V3 | ||||
|---|---|---|---|---|---|---|
| SJZT (n = 21) | GnRHa (n = 21) | OCs (n = 24) | SJZT (n = 21) | GnRHa (n = 21) | OCs (n = 24) | |
| Hot flush | 17 (81) | 16 (76) | 17 (71) | 2 (10) | 15 (71)* | 3 (13) |
| Insomnia | 7 (33) | 6 (29) | 5 (21) | 2 (10) | 5 (24) | 2 (8) |
| Arthralgia | 1 (5) | 1 (5) | 0 | 0 | 6 (29)* | 0 |
| Depression | 1 (5) | 1 (5) | 1 (4) | 0 | 1 (5) | 0 |
| Allergy | 0 | 0 | 0 | 1 (5) | 0 | 0 |
| Liver dysfunction | 0 | 0 | 0 | 1 (5) | 0 | 1 (4) |
*There were significant differences between the SJZT and GnRHa groups and between the GnRHa and OCs groups (P = 0.00). OCs: Oral contraceptives; GnRHa: Gonadotropin-releasing hormone analogs; SJZT: SanJieZhenTong.
One patient in the SJZT group had liver dysfunction with a slight increase in alanine aminotransferase and aspartate aminotransferase (AST) levels in V6, and another had an allergy with itchy erythema of the lower extremities in V4. One patient in the OCs group experienced liver dysfunction with a slight increase in AST levels in V6. All three patients discontinued assigned treatment. Furthermore, liver-protective treatments normalized the liver function of two patients, whereas the allergy symptoms were relieved spontaneously after the drug was discontinued.
Although conservative surgery for endometriosis is effective in symptom control, the recurrence rate is as high as 30%-50% within 3-5 years.[13] Therefore, long-term postoperative management is important. In this study, with the 9-month postoperative medical management, the 2-year recurrence rate was 4.2%-9.8% [Figure 2], which is lower than the general reported rate,[13] but similar to that reported by Li et al.[14] and Takamura et al.[15]
To the best of our knowledge, this is the first RCT showing the potential efficacy of an alternative medicine in the treatment of endometriosis. In terms of symptom control, the effectiveness of SJZT was comparable to that of GnRHa and OCs [Figure 3]. The possible underlying mechanism of action of SJZT has been partially described in previous publications.[16,17]
According to the theories of traditional Chinese medicine, endometriosis belongs to the category of "dysmenorrhea," "infertility," and "abdominal mass" with stagnant blood, qi, and body fluid as the pathogenesis,[16] which are specific indications of SJZT. SJZT consists of the original powder of four natural drugs: Resina Draconis, Panax pseudoginseng, Fritillaria thunbergii Miq, and coix seed.[18] The main bioactive constituents include ginsenoside Rg1, peimine, loureirin A, 7,4′-dihydroxyflavone, and pterostilbene,[19] which are associated with effects on inflammation, hormone regulation, cell adhesion, proliferation, and angiogenesis, which are associated with the development of endometriosis.[16] Du et al.[16] used a systematic network pharmacology analysis to show that SJZT was effective in treating adenomyosis through the phosphoinositide 3-kinase-Akt, nuclear factor-κB, and mitogen-activated protein-kinase signaling pathways. Furthermore, the authors reported that SJZT exerted anti-inflammatory effects by inhibiting nitric oxide and interleukin-6 production.[16] Zou et al.[17] found that SJZT reduced the size of endometrial explants in rats and the expression of vascular endothelial growth factor and tumor necrosis factor-α, which proved the efficacy of SJZT through experiments on rats.
The symptoms of endometriosis have negative consequences on daily life quality; personal relationships; and sexual,[20] digestive, and urinary functions,[21] which also require attention, in addition to symptom relief and recurrence prevention. Consequently, endometriosis should be considered a public health issue rather than just a disease of individuals.[21] The FSFI scores of the SJZT group were higher than those of the GnRHa and OCs groups [Figure 5c]. The rate of adverse events in patients in the Group SJZT was lower than that in the GnRHa group, but similar to that in the OCs group [Table 2]. GnRHa inhibits ovarian function and maintains low levels of estradiol through the hypothalamic-pituitary-ovarian axis to alleviate endometriosis-associated symptoms and reduce endometrial lesions.[22] Patients administered GnRHa treatment for >3 months often show menopausal symptoms, a high incidence of depression, and even sexual dysfunction[23] and could have a high risk of loss of bone density.[24]
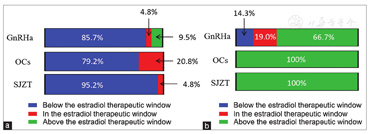
Barbieri[25] suggested a serum estradiol therapeutic window of 30-45 pg/mL where many endometriotic lesions might not be stimulated to grow and bone loss might be maximally avoided. In our trial, 85.7% (18/21) of patients in the GnRHa group required "add-back" therapy to maintain estradiol levels within the window [Figure 6a]. However, 71% (15/21) and 24% (5/21) of patients in the GnRHa group experienced hot flushes and insomnia, respectively [Table 2], which was not fully consistent with the findings of other studies on the effect of "add-back" therapy.[26,27]
Vercellini et al.[28] found a significant reduction in dyspareunia in patients treated with GnRHa. Nevertheless, sexual function should not only assess using the VAS score of dyspareunia but also involve the evaluation of sexual desire and arousal, orgasmic experiences, and vaginal lubrication. The FSFI questionnaire is one of the best-established tools for the assessment of sexual function in women with endometriosis.[29] Georgopoulos et al.[23] found that women with GnRH deficiency had significantly lower sexual desire than healthy women, which is consistent with the results of our trials. Similar to other hormonal drugs, OCs were associated with a decrease in FSFI, which was also similar to the findings of other RCTs reporting that OCs decreased sexual desire, arousal, and pleasure.[30,31] The possible underlying mechanism is that OCs decrease the levels of androgen,[32] which is involved in the aforementioned functions.[33] van Lunsen et al.[34] reported that maintaining physiological testosterone concentrations by coadministering dehydroepiandrosterone with OCs prevented these effects on sexuality. Although many studies have been conducted, the effects of OCs on sexual function have not been well studied and remain controversial.[35] SJZT is not a hormonal drug and does not have a significant influence on estradiol, liver and kidney function, and emotion, suggesting a potential for good compliance. The anti-inflammatory activity and abirritation might be the reason for the improved FSFI. Further studies on the effect of SJZT on sexual function are needed. Evaluation of digestive and urinary functions is necessary in patients with endometriosis, especially deep endometriosis. However, the CRADI-8 and UDI-6 scores were low in our trial, which indicated that the enrolled patients did not experience severe digestive and urinary system dysfunction, and there was no obvious change during the follow-up period [Figure 5a and Figure 5b]. However, this observation also indicates that the three therapies had few side effects associated with digestive and urinary function. Confirmation of the efficacy of SJZT on endometriosis-associated digestive and urinary discomfort might require a trial that enrolls more patients with related symptoms.
Other adverse events occurred during the follow-up period [Table 2]. After V3, one patient in the OCs group developed liver dysfunction, whereas one patient each in the SJZT group developed allergy and liver dysfunction. Although the side effects were resolved after withdrawal of the drug, these were warnings about the risk of liver damage with SJZT or OCs and allergy from SJZT.
There was no difference in the 2-year recurrence rate among the SJZT, GnRHa, and OCs groups, and the cost of GnRHa ($1526.9/person) was apparently higher than that of OCs ($ 661.7/person) and SJZT ($912.3/person). Furthermore, "add-therapy" involved an additional cost ($48.1/person) in the GnRHa group. The cost of SJZT was slightly higher than that of OCs, which may need to be balanced in terms of its effectiveness.A quarter to one-third of patients do not respond to OCs, have intolerances, or have contraindications.[36] For long-term management in these cases, SJZT might be a suitable alternative medication to OCs.
This study had some limitations that are worth mentioning. For the study design, we calculated the expected number of patients to be 140 per group, based on epidemiological requirements. However, because most patients in the GnRHa group exhibited significant side effects and were unable to complete the continuous nine injections, the enrollment was stopped after careful evaluation by the data safety monitoring board and the investigators. Nevertheless, those patients who were enrolled still received strict long-term follow-up, based on the original design of the trial. Although the sample size was small, we believe that the 2-year follow-up data hold certain clinical value. Therefore, we analyzed the current results and found that SJZT might have certain advantages in the treatment of endometriosis, especially in terms of sexual function. Based on the findings of this study, we improved the study design, revised the protocol, and have applied it to a new prospective, double-blind, RCT (ChiCTR1900027189). In conclusion, to the best of our knowledge, this is the first prospective RCT to show that SJZT is as effective as GnRHa and OCs in the postoperative management of endometriosis, with fewer adverse effects and the advantage of improving sexual function. Using the GnRHa regimen followed by SJZT may be an alternative strategy for patients with moderate or severe endometriosis. However, it would be difficult to draw a conclusion from a single institutional study, and therefore, a large-scale prospective multicenter study on the efficacy and safety of SJZT is warranted.
There are no conflicts of interest.






























