
Bird infections with highly pathogenic avian influenza A(H5N6) viruses have been identified since 2014. With very limited occasion, the virus could sporadically spilled over to infect humans. It has been recognized that all human infections were within southern region of Mainland China until the case reported here in Beijing in Aug. 2019. This was the first human case infected with highly pathogenic avian influenza A(H5N6) virus in northern China. The infection was confirmed by real-time RT-PCR assay. The whole genome sequences were obtained from clinical sample. Genetic characteristics of the virus were identified similar to those of previous avian influenza A(H5N6) viruses, retaining the main features of the avian influenza virus.

© 2020 Chinese Medical Association Publishing House. Published by Elsevier B.V. This is an open access article under the CC BY-NC-ND license (http://creativecommons.org/licenses/by-nc-nd/4.0/).
Highly pathogenic avian influenza (HPAI) A(H5) virus which derived from A/Goose/Guangdong/1/1996 lineage, has been causing epidemics in birds for over past two decades [1]. In Mainland China, H5N1 has remained as main subtype of HPAI H5 until about 2013 when H5N2/N5/N6/N8 subtypes were identified [2]. The HPAI A(H5) virus circulates within poultry and sporadically spills over to human and causes infection with severe symptoms. So far, both HPAI H5N1 and H5N6 subtypes have been reported to infect humans [3,4]. HPAI H5N6 became dominant H5 subtype causing human infections after its emergence in Mainland China, consistent with the prevalence of viruses in poultry [5,6]. Up to July 2019, 23 human cases of HPAI H5N6 virus infection have been reported to the World Health Organization (WHO) and the mortality rate of the infection reached to nearly 70% (16/23). All the cases occurred exclusively in southern China (Fig. 1). However, on August 17, 2019, the first case of human infection with HPAI H5N6 virus was laboratory confirmed in Beijing located in northern China.


The patient was a 59-year-old female who came to Beijing from Guangdong in May 2019. On August 6, the patient had a fever of about 38 °C but no other symptoms. The patient was hospitalized on August 8, and then was transferred to ICU on August 10 due to occurrence of dyspnea. On August 14, Oseltamivir (150 mg q12H) was given to the patient since sputum nucleic acid test showed positive of influenza A virus. Also, alveolar lavage fluid specimen collected on August 16 showed H5N6 positive in real-time RT-PCR testing. Both sputum and alveolar lavage fluid specimens collected on August 19 and 22 respectively, were negative for influenza A virus. This was the first human case identified in Northern China (Fig. 1). Epidemiology investigation showed that although the patient has no history of direct exposure to live poultry exposure, the patient has cooked the fresh chilled poultry (chicken and duck) given by friend from Hunan Province in July 2019.
The full genome sequences of the HPAI H5N6 virus were obtained by direct sequencing from respiratory specimens collected on August 16, using MiSeq high-throughput sequencing platform (Illumina, Inc. San Diego, California). The hemagglutinin (HA) gene possess a multiple basic peptide, PRERRRKR↓G, in the cleavage site, indicating the presence of HPAI virus. BLAST searching on the Global Initiative on Sharing All Influenza Data Database showed that each segment of the human virus met the highest similarity (98.3%-99.4%) with those of previously identified H5N6 viruses (Table 1).
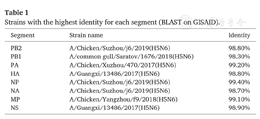
Strains with the highest identity for each segment (BLAST on GISAID).
Strains with the highest identity for each segment (BLAST on GISAID).
| Segment | Strain name | Identity |
|---|---|---|
| PB2 | A/Chicken/Suzhou/j6/2019(H5N6) | 98.80% |
| PB1 | A/common gull/Saratov/1676/2018(H5N6) | 98.30% |
| PA | A/Chicken/Xuzhou/470/2017(H5N6) | 99.20% |
| HA | A/Guangxi/13486/2017(H5N6) | 98.80% |
| NP | A/Chicken/Suzhou/j6/2019(H5N6) | 99.40% |
| NA | A/Chicken/Suzhou/j6/2019(H5N6) | 98.70% |
| MP | A/Chicken/Yangzhou/f9/2018(H5N6) | 99.10% |
| NS | A/Guangxi/13486/2017(H5N6) | 98.90% |
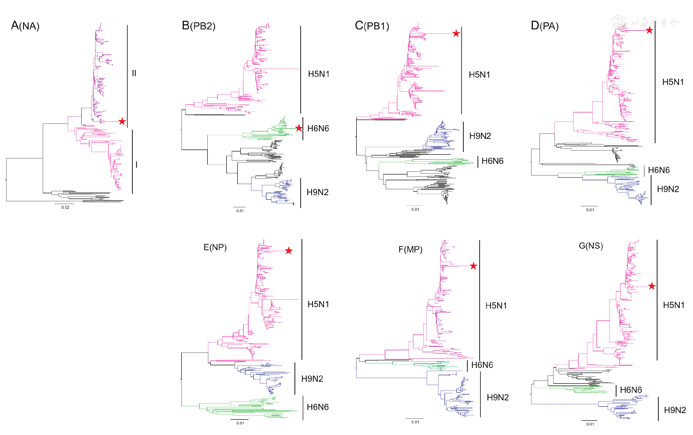
Phylogenetic analyses were performed with selected sequences to determine the gene cassette of the human virus. Approximate maximum likelihood (ML) phylogenetic trees for each gene segment were constructed using MEGA software version 10.0.5 with the general reversible GTR + gamma model. The HA gene belonged to Clade 2.3.4.4II (Fig. 2). The neuraminidase (NA) gene belonged to the subclade II with the deletions at 59–69 sites (Fig. 3A). The polymerase basic protein 2 (PB2) was derived from H6N6 duck viruses (Fig. 3B). While other internal genes, including polymerase basic protein 1 (PB1), polymerase acidic protein (PA), nucleocapsid protein (NP), the matrix proteins (MP) and the nonstructural proteins (NS) were derived from Clade 2.3.2.1c H5N1 viruses (Fig. 3C–G). Similar genotype of H5N6 viruses had been documented to circulate in Mainland China since 2014 [5,6] and infected human in 2015 in Guangdong, China [5].
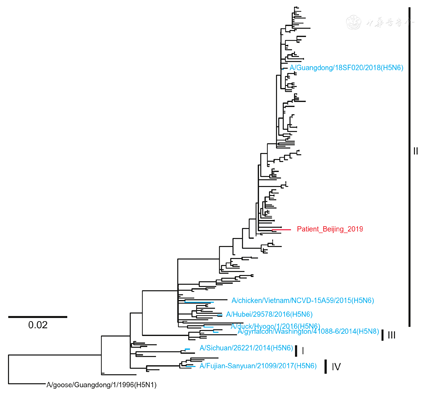


We further examined the molecular markers for drug resistance, virulence and mammalian adaption of the H5N6 virus. The reported NA inhibitor-resistant mutations (E119D, A246V, R292K and R371K) were not identified [7], so were the amantadine-resistant mutations (L26F/S, V27A, A30T/V/S, S31N/G and G34E). The receptor binding site in HA contained 226Q and 228G (H3 Numbering), indicating its avian-like receptor properties [8]. The well-known mammalian-adapting mutations (E627K, D701N and Q591K) in PB2 were not changed. The virus carried mutations that have been reported to increase virulence in mice, including N30D and T215A in MP1 [9] and P42S in NS1 [10]. We also compared the HA1 sequence with the closest candidate vaccine virus, A/Guangdong/18SF020/2018(H5N6) (Fig. 2). Nine mutations, V51I, N54D, A86V, E186D, D189N, A214V, T215N, Y252F, and E268R (H5 Numbering), were found. Whether the antigenicity has changed, the virus needs to be isolated and tested.
The newly emerged HPAI H5N6 virus in human suggested their sustainable threaten to the public health and pandemic potential. As the H7N9 cases was significantly decrease by vaccination of poultry [11], vaccination of HPAI H5 was not such effective. Close monitoring of the H5N6 viruses is needed both in human and poultry.
This study was supported by the National Mega-projects for Infectious Diseases (2017ZX10303401-004). We thanks CDCs in Beijing for sample collections and field investigation.
The authors declare that there are no conflicts of interest.






























