
Antiphospholipid antibody syndrome is an autoimmune disorder that primarily affects reproductive age women and poses significant obstetric complications. Here we review the pathogenesis, diagnosis, obstetric complications, and management in women with antiphospholipid antibody syndrome.

Copyright © 2019 The Chinese Medical Association, published by Wolters Kluwer Health, Inc. This is an open access article distributed under the terms of the Creative Commons Attribution-Non Commercial - No Derivatives License 4.0(CCBY - NC - ND), where it is permissible to download and share the work provided it is properly cited.The work cannot be changed in any way or used commercially without permission from the journal.
Antiphospholipid antibody syndrome (APS) is an autoimmune condition characterized by the presence of antiphospholipid antibodies (aPL) along with certain clinical features.1 The condition is prevalent among reproductive age women with as high as 75% of the cases occurring in this group.2 Thus, it is important to be aware of the diagnosis, treatment, and clinical outcomes for pregnant women with APS.
Despite extensive studies in unraveling the mechanism of APS, the precise pathogenesis of the disease remains unclear. Although the presence of specific autoantibodies of the IgG or IgM isotype, detected by enzyme-linked immunosorbent assay (ELISA) for anti–β2-glycoprotein I (β2G2PI) or anticardiolipin antibodies (aCL) or by lupus-anticoagulant assays is essential for the diagnosis, it is unclear (1) why some individuals develop these antibodies, and (2) why some who developed these antibodies have clinical manifestations while others do not. The clinical features of APS are vascular thrombosis and/or obstetric complications likely resulting from vascular thrombosis.
What triggers vascular thrombosis remains unclear. The "two hit" hypothesis suggests that a state of systemic inflammation and tissue damage results from triggers such as oxidative stress, surgery, trauma, or infections leading to endothelia cell damage as the "first hit." 3 This exerts different effects on endothelial cells, monocytes, platelets, and complement.4,5 Binding and activation of these cell types causes an increased expression of adhesion molecules, secretion of cytokines, and production of arachidonic acid metabolites.6 aPL may also participate in oxidant-mediated injury to vascular endothelium or bind to perturbed cells that lose their regular membrane symmetry and express anionic phospholipids on their surface.6 The interaction of antibodies with clotting regulation such as prothrombin, factor X, protein C, and plasmin might hinder inactivation of procoagulant factors and impede fibrinolysis. In pregnancy, placental thrombosis and fetal loss may result from interference with annexin A5, a natural anticoagulant.5 Abnormalities in placentation leading to pregnancy loss may result from antibodies binding leading to a reduction of human chorionic gonadotropin secretion or triggering an inflammatory response resulting in trophoblast damage.5
APS is suspected when patients have obstetrical complications or vascular thrombosis.7 Clinical suspicion prompts laboratory testing, which is required as part of the diagnosis criteria. The revised Sapporo criteria for clinical and laboratory criteria of APS are listed in Table 1.

Updated Sapporo criteria for the diagnosis of antiphospholipid antibody syndrome (APS)
Updated Sapporo criteria for the diagnosis of antiphospholipid antibody syndrome (APS)
| Diagnosis: Vascular thrombosis and/or pregnancy morbidity plus laboratory criteria | ||
|---|---|---|
| Clinical criteria | ||
| Vascular thrombosis | One or more clinical episode of arterial, venous, or small vessel thrombosis in any tissue or organ, which is confirmed by objective criteria (imaging, Doppler, or histopathology) | |
| Pregnancy morbidity | 1. One or more unexplained deaths of a morphologically normal fetus ≥10 weeks gestation with normal fetal morphology documented by ultrasound or on fetal examination. | |
| 2. One or more premature births of a morphologically normal neonate before 34 weeks gestation due to eclampsia or severe preeclampsia or features consistent with placental insufficiency (abnormal Doppler flow, abnormal fetal testing, intrauterine growth restriction, oligohydramnios) | ||
| 3. Three or more unexplained consecutive spontaneous abortions before the 10th week of pregnancy | ||
| Laboratory criteria* | ||
| 1. Lupus anticoagulant present in plasma | ||
| 2. Anticardiolipin antibody of IgG and/or IgM isotype in serum or plasma, present as > 40 GPL or MPL or > 99th | ||
| 3. Anti-B2 glycoprotein-I of IgG and/or IgM isotype in the serum or plasma (in titer >99%) | ||
* At least one antibody on two occasions 12 weeks apart.
APS can be primary when the patient does not have any history of underlying disease or laboratory abnormalities, or secondary when it is associated with other diseases or conditions.1 Secondary APS can be founded in association with other autoimmune conditions such as systemic lupus erythematosus (SLE), rheumatoid arthritis, Sjogren syndrome, systemic sclerosis, diabetes mellitus, Crohn’s disease, autoimmune thyroid disease, malignancies of the ovary and cervix, lymphoma or leukemia, drug induced as with oral contraceptive pills or in infectious disease such as syphilis or HIV.1
The clinical criteria of APS encompass either vascular or obstetric complications. Vascular thrombosis is defined as at least one episode of a confirmed arterial, venous, or small vessel thrombosis. Obstetrical morbidity includes at least one of the following: (1) one or more unexplained demise of a morphologically normal fetus at or beyond 10 weeks gestation, (2) one or more premature births of a morphologically normal neonate at or before 34 weeks of gestation caused by severe preeclampsia or severe placental insufficiency, or (3) at least three unexplained, consecutive miscarriages of less than 10 weeks of gestation.1
In addition to the above clinical criteria, the present diagnosis criteria for APS require one of the three laboratory assays to be positive: (1) aCL, (2) antiβ2GP1, or (3) lupus anticoagulant. Women with triple antibody positivity are at higher risk of poor obstetrical outcomes than those with a single positive antibody.8
Lupus anticoagulant is believed to be the assay of choice in detection of clinically relevant aPL.8,9,10 Testing for lupus anticoagulant is ideally performed before treating a patient with anticoagulation and is interpreted as either present or absent.2 Testing for lupus anticoagulant has historically been based on a series of three consecutive assays: screen, mixing, and confirm.10 The screening assay is performed first that identifies a prolongation of clotting assay such as sensitive activated partial thromboplastin time and dilute Russell’s viper venom time.2,10 The mixing assay evaluates for the possibility of a factor deficiency leading to prolongation.10 If the clotting time is caused by a factor deficiency, the addition of normal plasma results in normal clotting time on repeat testing. However, if lupus anticoagulant is present, clotting time remains prolonged despite the addition of plasma.2 Finally, the confirm assay identifies the inhibitor of coagulation as phospholipid dependent by neutralizing the prolongation with extra phospholipids.
aCL are detected using enzyme ELISA.2,11 aCL of immunoglobulin G (IgG) and immunoglobulin M isotype are part of the diagnostic criteria for APS. Historically, interlaboratory consensus on assay interpretation was difficult due to the reporting of semiquantitative results (ie, negative, low, medium, or high). However, agreement has improved with the use of standard sera from the Antiphospholipid Standardization Laboratory in Atlanta, Georgia.2,11 Results for aCL are reported in international standard units, designated as GPL for IgG phospholipid and MPL for IgM phospholipid. Current clinical criteria designate a positive result as a medium or high titer, which correlates to greater than 40 GPL or MPL, or greater than the 99th percentile.2
β2GP1 are risk factors for pregnancy complications and thrombosis.12 These antibodies are detected using ELISA and reported as "SGU" for IgG and "SMU" for IgM. A positive test result is reflected by a titer greater than the 99th percentile.12
Many other antibodies have been shown to be directed to other proteins of the coagulation cascade (ie, prothrombin and/or phosphatidylserine-prothrombin complexes) or complexes other than cardiolipin or to some domains of β2GP2.10 Hence, whether these isotype tests or IgA aPL should be a part of the APS diagnostic algorithm is a subject of hot debate.10
Though IgA aCL may be similar to IgG in terms of thrombogenicity and cofactor requirement, controversies exist regarding their clinical associations that are attributable to study design and the use of various nonstandardized assays.13 The task force recommends measurement of IgA only in cases where IgG and IgM aCL are negative and there is a very strong suspicion for APS.13 The task force has the same recommendation for IgA anti-β2GP1, in which these antibodies should be testing in the presence of clinical signs and symptoms of SLE and/or APS, particularly when other aPL tests are negative. Well-designed studies are needed for the evaluation and comparison of diagnostic assays. The task force also recommends that further investigation should be carried out on the role of IgA β2GP1 and the pathogenesis of APS.13
The 14th International Congress on Antiphospholipid Antibodies Task Force met in Rio de Janeiro, Brazil in 2013 to review laboratory diagnostics and trends, while the 13th Annual Congress reviewed "noncriteria" aPL tests in Galveston, TX, in 2010. The 13th Annual Congress acknowledges that several "noncriteria" aPL may have clinical significance, but that current level of evidence did not warrant any changes to the current criteria.13 The task force recommendations from the 13th Congress acknowledge the possible clinical associations of other antibodies in APS, although it is clear that further research through well-designed studies and assay development to eradicate technical problems and inconsistencies need to be conducted before expanding upon the current laboratory criteria for APS.13 The findings of the 14th Congress are beyond the scope of this article, although they may be reviewed for diagnostic dilemmas. As it stands, the revised Saparro criteria, only lupus anticoagulant, anti-β2GP1, and aCL are included in the diagnosis criteria. It is true that several other autoantibodies have been suggested to be relevant to APS, although their clinical utility and diagnostic value remain elusive at the present time.10
aPL can be transiently positive due to other conditions such as a viral syndrome. Thus, it is recommended that a positive test should be confirmed at least 12 weeks after the initial positive test to confirm its presence. Once a diagnosis has been confirmed, repeat testing is unnecessary, although revaluation can be considered in certain clinical situations.14
The APS clinical criterion includes three or more unexplained consecutive spontaneous abortions before the 10th week of gestation. However, many factors can contribute to early miscarriage and recurrent miscarriages, including genetic abnormalities as the most common cause, in addition to the association with aCL and/or lupus anticoagulant antibodies.15 The prevalence of both antibodies varies depending on the study, with one reported incidence 1.0%–2.2% and 1.2%–3.8%, respectively, in normal pregnant populations compared to 13.9%–21.0% and 2.8%–14.6% in recurrent pregnancy loss.15 aPL are also found in about 50% of cases of pregnancy loss beyond 10 weeks of gestation.15 The association between stillbirth and APS is less defined due to multiple possible contributing factors such as systemic lupus erythematosus or renal disease.1,15 One study assessed aPL in a cohort of women with stillbirth and demonstrated that aCL and anti-β2 glycoprotein antibody were positively associated with stillbirths (beyond 20 weeks). However, lupus anticoagulant was not analyzed in the study.16 Triple positivity confers a much higher rate of fetal loss compared to when two tests are positive.1,17 What contributes to the significant variations are as follows: (1) Most of these studies are retrospective and published before 2000. (2) A few studies tested all three aPL. (3) Many of these studies had a low antibody titer cutoff. (4) A few studies had confirmation test of aPL.18,19
Patients with APS are at increased obstetric complications. Recent prospective multicenter study showed that patient with primary APS and multiple antibody positive results had a significantly lower live birth rate compared with women with only one antibody positive test results. These patients were also at increased risk of preeclampsia, intrauterine growth restriction, and stillbirth.20 Therefore, in the workup of etiology of preeclampsia with severe features requiring preterm delivery before 34 weeks of gestation and early onset of intrauterine growth restriction suspicious of placental insufficiency, aPL should be tested in these patients. At least 20% of patients with severe preeclampsia before 34 weeks of gestation have at least one positive antibody compared to 6% of patients with late-onset preeclampsia.1
Patients with APS may require lifetime anticoagulation and regular follow-up with hematologist, especially for those who have had thromboembolism Fig. 1. Contra-ception and family planning are critical for reproductive age women with APS. Estrogen-containing contraception method is contraindicated in patient with APS due to the increased risk of vascular thrombosis. Some anticoagulation medication such as warfarin may have teratogenic effect if exposed during early organogenesis period and should be switched to a better medication prior to conception and continue at least for the duration of first trimester. Therefore, it is important for women with APS to follow up closely with her obstetrician/gynecologist for an ideal contraception method, and consult a Maternal Fetal Medicine expert before they are planning a family.
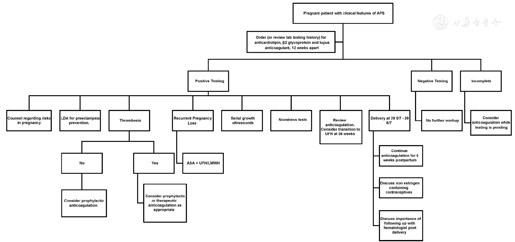

Prior to conception, the patient should also complete baseline laboratory testing to evaluate for underlying anemia and thrombocytopenia. A baseline renal function including urinalysis, serum creatinine, 24-hour urine for creatinine clearance, and total protein should also be performed due to the risk of preeclampsia.6
Once pregnant, the goal of treatment is to optimize maternal and neonatal outcomes. The patient should seek prenatal care as soon as the pregnancy is confirmed. Patients with a history of recurrent pregnancy loss may benefit from prophylactic low-molecular-weight heparin or unfractionated heparin in addition to low-dose aspirin as this has shown to reduce pregnancy loss by 54%.21,22 Anticoagulation in pregnancy should be individualized. Patients with APS who had a thrombotic event should be administered prophylactic anticoagulation throughout pregnancy, and continue 6 weeks postpartum.2 For patients with APS and recurrent vascular embolism, intermediate or adjusted dosage of anticoagulation should be considered. Studies are more limited in women who have not had a thrombotic event; however, either clinical surveillance or prophylactic heparin during antepartum and 6 weeks postpartum can be considered.2 Heparin therapy can include many risks such as heparin-induced thrombocytopenia or osteoporosis. Laboratory evaluation of platelets should be obtained prior to starting therapy and weekly in the first 3 weeks after starting injections.16 Patients should also take at least 1 g of calcium daily and walk for 1 hour a day to avoid osteoporosis.6,20 Additionally, the American College of Obstetricians and Gynecologists recommends to start low-dose aspirin at 12–28 weeks, ideally before 16 weeks of gestation to decrease the risk of preeclampsia.23
The patient should be educated regarding the signs and symptoms of preeclampsia and blood pressure should be monitored at prenatal visits per routine guidelines. A routine anatomical survey should be performed between 16 and 18 weeks of gestation, and the patient should have serial ultrasounds to monitor for fetal growth.6 Antepartum testing is recommended in the third trimester due to the risk of growth restriction and stillbirth.2
For a pregnant patient who is on anticoagulation, coordinated team efforts between MFM, anesthesiology, and hematology are essential for clinical management. Timing delivery between 39 0/7–39 6/7 weeks of gestation to control anticoagulation discontinuation should be considered.24 If a patient is on low-molecular-weight heparin, conversion to unfractionated heparin should be considered due to the risk of spinal or epidural hematoma with regional anesthesia.24 The Society of Obstetric Anesthesia and Perinatology provides clinical guidance regarding thromboprophylaxis and neuraxial anesthesia considerations. The society also recommends that each institution has a protocol for anticoagulation management and eligibility for neuraxial anesthesia.25 Women who are receiving prophylactic LMWH or unfractionated heparin dose exceeding 7500 units should discontinue anticoagulation at least 12 hours before scheduled induction. Patients who are on therapeutic or adjusted dose, low-molecular-weight heparin could be transitioned to adjusted dose unfractionated heparin by 36 weeks of gestation due to the shorter half-life or could be induced within 24 hours of discontinuing therapy if appropriate, depending upon clinical scenario.24,25 Pneumatic compression devices should be used during labor and delivery until the patient is ambulatory.24,25
Contraception options containing estrogen should be avoided in the postpartum period due to the increased risk of venous thromboembolism.2,24 Patients should be counseled on the long-term risks of stroke, thrombosis, and development of SLE and should be followed closely by a medical or hematological specialist after delivery.2
None.






























