
Systemic lupus erythematosus (SLE) is an autoimmune disease with extreme heterogeneity and potentially involvement of any organ or system. Numerous unanswered questions and challenges in SLE always prompt further exploration. In 2019, great progress in various aspects of SLE emerged. Both the classification criteria and management recommendation for SLE were updated. New promising medications have been widely developed and tested, although subsequent clinical studies are warranted. As an emerging number of most notable studies in SLE were published in both clinical area and basic research in 2019, we aim to summarize the highest quality data on SLE regarding novel insights of pathogenesis, updated recommendations, hot-spot issues on clinical manifestations, new understanding of disease prognosis, and most importantly, the therapeutic advances in SLE in this review.

Copyright © 2020 The Chinese Medical Association, produced by Wolters Kluwer, Inc. under the CC-BY-NC-ND license. This is an open access article distributed under the terms of the Creative Commons Attribution-Non Commercial-No Derivatives License 4.0 (CCBY-NC-ND), where it is permissible to download and share the work provided it is properly cited. The work cannot be changed in any way or used commercially without permission from the journal.
Systemic lupus erythematosus (SLE) is a chronic, prototypic autoimmune disorder which may affect almost any organ or system.[1] The high heterogeneity of SLE has been long recognized by clinicians and scientists. With the advance in early detection and proper management, disease burden and mortality of SLE has dramatically declined, for instance, a 10-year survival from 63.2% in the 1950s to 95% in the modern era.[2,3] Year 2019 was definitely a landmark for SLE. Two novel recommendations launched by the European League Against Rheumatism/American College of Rheumatology (EULAR/ACR) were published. Moreover, belimumab, a human IgG1λ monoclonal antibody against B-lymphocyte stimulator (BLyS), also the first biological drug to treat SLE was approved by China Food and Drug Administration in 2019. In this article, the most notable clinical and basic researches in the field of SLE published in 2019 are reviewed, with focus on recommendations, pathogenesis, clinical manifestations, disease prognosis, and therapeutic advances.
As a starting point, a PubMed search was performed for articles published between January 1, 2019 to December 31, 2019, using the search terms "lupus [All fields]," with the limit of English language. The search resulted in 3902 articles, including 590 reviews, 25 clinical trials, 429 case reports, and other clinical and basic studies in SLE. All the titles of publications and abstracts were subsequently reviewed. The most relevant literatures with high quality about the aforementioned fields of SLE were eventually selected and reviewed.
With the effort of EULAR and ACR committees, the revised classification criteria[4] and updated management recommendations[5] for SLE were freshly proposed. Unlike with ACR-1997[6] and Systemic Lupus International Collaborating Clinics-2012 classification criteria,[7] EULAR/ACR-2019 criteria are a score-based system. There are ten hierarchical domains (seven clinical and three immunological) consisting of a total of 22 criteria with distinct weights in this new classification criteria. Positivity of anti-nuclear antibodies (ANA) is the entry criterion, and then an individual patient with a total score of 10 or higher would be classified as SLE. Of note, in this situation, the patients with negative ANA would not be allowed for classification of SLE. Thus, high-quality ANA testing is extremely important and it calls for more efforts to standardize the test.
An updated recommendation for the management of SLE was proposed in 2019. Over a decade has passed since the previous version was published in 2008. Notably, the recommendation 2019 strongly emphasizes to minimize disease activity with remission or low disease activity as a principle goal of therapy. This reflects the art of treat-to-target strategy in SLE. Correspondingly, a number of studies demonstrated that achieving remission or low disease activity would significantly reduce damage accrual and improve health-related quality of life in SLE.[8,9,10] In addition, belimumab, the first approved biological drug for SLE, was newly recommended to patients with extra-renal disease, inadequate control by first-line treatments, and inability to taper glucocorticoids daily dose to acceptable levels (ie, prednisone 7.5 mg/d). Efficacy of hydroxychloroquine (HCQ) in lupus has been very well established. Last year there were also abundant discussions regarding the recommended dosage of HCQ (no more than 5 mg/kg daily) in the new recommendations. Whether the lower dose HCQ processes comparable clinical effects to the previously recommended 6.5 mg/kg daily still needs to be confirmed.[11,12] Measuring HCQ concentration in serum can be helpful to detect patient’s adherence, but the concentration-adjusted dosing therapy has not been proven superiority yet. Further studies are urgently required. Screening for HCQ retinopathy should be done before administration, repeated at 5 years, and then every year during HCQ treatment, according to the recommendation.
SLE is characterized by immune dysregulation and aberrant production of auto-antibodies. Although the precise pathogenesis in SLE still needs to be elucidated, abnormalities in activation of innate and adaptive immune system are well acknowledged.[13,14] The type I interferon (IFN) family of innate immune cytokines contributes to the aberrant immune functions of SLE. Recent work on Science revealed a novel mechanism between mitochondrial DNA (mtDNA) and autoimmunity.[15] Short mtDNA fragments released by oxidative stressed mitochondria could enter the cytosol via the pores formed by oligomerization of the mitochondrial voltage-dependent anion channel, and then induce type I IFN production and promote lupus-like disease. Type I IFN genes were also highly expressed in neutrophils. The emerging roles of neutrophils and neutrophil extracellular traps in SLE inflammation and autoimmunity were well summarized by clinical scientists.[16,17] In a paper published in Nature Medicine, Caielli et al[18] identified a novel T cell subset in SLE, CXCR5-CXCR3+PD1hiCD4+ T peripheral helper cells, which could activate B cells in a unique manner and promote autoantibody development. In 2019, an excellent research conducted by Chinese scientists was published on Cell. In SLE patients, augmented protein kinase phosphorylation and circular RNAs (circRNAs) reduction were observed. Functional study showed that endogenous circRNAs tended to form imperfect RNA duplexes and acted as inhibitors of double-stranded RNA-activated protein kinase related to innate immunity, thus providing a connection between circRNAs and SLE.[19] Immunotherapy by reinforcing functional T lymphocytes, like immune checkpoint inhibitors (ICIs), has led tremendous successes in the treatment of advanced cancers, but immune-related adverse events caused by ICIs were diverse and should be noted. Raschi et al[20] analyzed the Food and Drug Administration (FDA) Adverse Event Reporting System to verify whether SLE was reported with ICIs treatment, and not surprisingly, 18 lupus cases related to ICIs (especially programmed cell death-1/programmed cell death ligand-1 inhibitors) were identified. This further confirmed the important roles of T lymphocytes in the pathogenesis of SLE. Besides, Arnaud et al[21] even identified 118 drugs associated with drug-induced lupus (DIL), and 42 of them had not been previously reported, suggesting the necessity of revisiting the spectrum of DIL. To know more about pathogenesis of SLE may help us to improve recognition of lupus development as well as management, and be beneficial for the new drug discovery.
LN is a severe complication which affects up to about 50% of SLE patients. Although a decrease in the severity of LN has been reported during recent decades, the disease burden should not be underrated. A study based on French nationwide database[22] showed that 10.1% of the 6011 patients who had no chronic kidney disease (CKD) at baseline eventually developed CKD, while 33.15% of the 428 patients with CKD at baseline eventually developed end-stage renal disease (ESRD). Kidney transplantation is now a well-established option for patients with LN-ESRD. Nationwide cohort study in US confirmed that renal transplantation was associated with a survival benefit in LN-ESRD, primarily due to reduced deaths from cardiovascular disease and infection.[23] One of the most advanced researches of LN in 2019 was the discovery of immune cell landscape in kidney.[24] By using single-cell RNA sequencing, scientists mapped 21 immune cell clusters in LN kidney, including B cells, T cells, natural killer cells, myeloid cells, and epithelial cells. Gene expression of immune cells in urine revealed a high correlation with the gene signature of kidney immune cells, which makes it possible to replace kidney biopsies by urine test in the future. Similarly, Der et al[25] identified molecular signatures that could potentially be used to predict treatment responses in LN by single-cell RNA sequencing of kidney and skin biopsy samples. Skin may be served as another surrogate source of kidney biopsy.
NP-SLE, collectively referred to as neurologic and psychiatric involvement in SLE, is highly diverse with a broad spectrum of presentations (range from subtle cognitive dysfunction to acute confusion states, psychosis, stoke). A comprehensive review published in Nat Rev Rheumatology 2019 summarized the frequencies of 19 neuropsychiatric manifestations that can occur in SLE (12 relating to central nervous system, seven to peripheral nervous system).[26] There has been a large body of work on central nervous system (CNS) involvement in SLE patients, but involvement of peripheral nervous system in 7.6% of SLE patients reported from a multi-ethnic, prospective inception cohort study,[27] also reduced patients’ health-related quality of life. Although research interest in NP-SLE has been growing, the diagnosis of NP-SLE remains full of challenges. Autoantibodies would be the most promising biomarkers for the diagnosis. The Swiss SLE Cohort Study Group demonstrated that antibodies against components of nervous system were found in 13% (23/174) of total SLE populations, and anti-myelin oligodendrocyte glycoprotein antibody was significantly associated with NP-SLE.[28] Besides, some classic antibodies, such as anti-phospholipid, anti-ribosomal P, and anti-aquaporin 4 antibodies are implicated in NP-SLE. Nevertheless, no gold standard has been established for the diagnosis of NP-SLE so far. With the rapid development of neuroimmunology, an emerging number of biomarkers in the field of NP-SLE will come out.[29] In clinical practice, discrimination between NP-SLE and CNS infections in SLE patients is always important and sometimes challengeable. A simplified scoring system integrated with eight key factors was proposed by the Peking Union Medical College Hospital to assist distinguishing CNS infections from NP-SLE.[30] Fulfilling four or more criteria showed a superior ability in predicting CNS infection with sensitivity of 85% and specificity of 84.2%. Of note, the proposed scoring system still needs to be further validated in large prospective cohorts and is worthy of expectation.
In general, inflammatory musculoskeletal symptoms are common in SLE, varying from inflammatory arthralgia to frank synovitis and Jaccoud arthropathy as a result of capsular laxity. With the advances of modern imaging techniques in rheumatology, ultrasound has been more and more widely used to detect musculoskeletal abnormalities, as well as to assess the disease activity. Recently, a large observational study revealed that inflammation was detected by ultrasound in 27% of SLE patients with arthralgia, however, with no clinical arthritis. More importantly, the ultrasound-only inflammation in the joints was proved to be associated with worse clinical symptoms and serology.[31] An interesting study comparing the ultrasound findings and clinical assessment in reflecting the therapeutic response in SLE patients with musculoskeletal manifestations revealed that ultrasound was better than clinical assessment due to the nature of being more objective. Thus, most clinical trials based on the traditional clinical outcome measurements (SLE disease activity index [SLEDAI] and SLE responder index-4 [SRI-4]) may underestimate the efficacy of treatment in SLE.[32] The role of sub-clinical synovitis/tendonitis on ultrasound in assessing disease activity and guiding treatment needs to be further investigated.
Hematological and cardiovascular systems are commonly involved in SLE. A meta-analysis published last year revealed SLE patients with positive anti-phospholipid antibodies had a higher risk in developing thrombocytopenia than those patients with negative anti-phospholipid antibodies (odd ratio 2.48, 95% confidence interval [CI] 2.10-2.93).[33] Data from hospitalized patients in US suggested that SLE was associated with a higher prevalence of atherosclerotic cardiovascular disease compared to those non-SLE (25.6% vs. 19.2%, respectively).[34] Pulmonary arterial hypertension (PAH), a devastating cardiopulmonary complication of SLE, was identified as the third leading cause of mortality in SLE.[35] A nationwide cohort study indicated that 2.13% of 15,783 incident SLE patients developed PAH, mostly (about 70%) during the first 5 years after SLE onset. The overall 1-, 3-, and 5-year survival rates after PAH diagnosis were respectively 87.7%, 76.8%, and 70.1%.[36] Risk factors in identifying PAH from SLE were widely investigated last year.[37,38] In addition, some scholars proposed a novel category of SLE-PAH, vasculitic subtype and vasculopathic subtype according to the systemic manifestations and disease activity.[39] Theoretically, treatment for the patients in the two distinct clusters might be markedly different, which reflected the hope of precision medicine in SLE-PAH.
Since SLE is a fairly complicated autoimmune disease which potentially affects any organs, the accurate assessment of its disease activity remains a pending task. A novel SLE disease activity score (SLE-DAS) system was proposed based on outpatients and provided a more accurate identification of clinically meaningful changes over time compared with SLEDAI-2K system.[40] Subsequently, SLE-DAS was validated in an independent cohort from Latin American as a useful tool in measuring activity in SLE patients; however, is controversial in those severe patients.[41] Thus, this new tool needs to be externally validated in more well-designed cohorts.
Although prognosis of SLE patients has been improved substantially from 5-year survival rate of approximately 50% in 1950s to over 90% in the early 2000s,[42] SLE is still life-threatening and a major cause of premature death. In 2019, a large, long-term follow-up cohort study revealed that patients with SLE were three times more likely to die from any cause compared with patients without lupus.[43] Of note, standardized mortality ratio (SMR) was particularly higher in patients younger than 40 years old, which underlined more attention needs to be paid in this sub-population. In addition, a huge racial disparity in mortality associated with SLE was demonstrated by Lim et al.[44] Compared with Caucasian SLE patients, cumulative SLE mortality was significantly higher among blacks (SMR 3.34 vs. 2.43, respectively).[44] This high burden of mortality in SLE may be partially interpreted by increased risks of multiple comorbidities. In a latest study, UK scholars noticed that SLE was associated with greater risk of any comorbidity at and after diagnosis.[45] Furthermore, comorbidities at SLE diagnosis accounted for 27.6% of the apparent difference in mortality between SLE patients and matched controls, which calls for a thorough search for comorbidities in SLE patients. The unchanged trend of premature mortality in SLE highlights critical unmet need for improved and optimized management of SLE, especially for new treatments.
During the past decades, a large number of novel drugs in double-blind clinical trials for the treatment of SLE failed and disappointed clinicians. Belimumab, the first drug approved for SLE following assessment in a randomized clinical trial (RCT), put an end to the dilemma of unavailability of new medication for SLE for more than half a century. In 2019, a study based on 13-years’ safety and efficacy data of belimumab plus standard therapy demonstrated that belimumab was well tolerated with no new safety concerns, and efficacy was further improved.[46] A propensity score-matched comparative analysis conducted by Urowitz et al[47] revealed that belimumab-treated group experienced significantly less progression of organ damage compared with patients receiving standard therapy (harzard ratio 0.391; 95% CI 0.253-0.605; P < 0.001). These results highlighted the efficacy and tolerability of belimumab as a novel therapeutic agent for SLE. Undoubtedly, the approval of belimumab for the treatment of SLE and the recognition that clinical trial design can be improved, have kindled hopes for the exploration of novel medications for SLE. Several new approaches targeting B cells, cytokines, or intracellular signaling pathways, are fiercely under investigation.
Activation of type I IFN has been recognized as a central pathogenic mediator of SLE, thus blocking IFN or its receptors has been considered to be top priorities for the treatment of SLE. One of the most anticipated lupus trial to date which was newly published on N Engl J Med, reported that anifrolumab, a human monoclonal antibody to type I IFN receptor subunit 1, was associated with a significantly higher response rate at week 52 compared with placebo.[48] IFN-α-kinoid (IFN-K) is another option in blocking IFN pathway. IFN-K, a vaccine composed of inactivated recombinant human IFN-α2b, acts by inducing antibody production against all IFNα subtypes. A phase IIb trial involving 185 patients with mild-to-moderate SLE showed IFN-K significantly reduced the IFN gene signature with an acceptable safety profile, and allowed more steroid reduction and attainment of lupus low disease activity state.[49] Nevertheless, the trial failed to achieve the primary endpoint, which inspired more thoughts on the choice of the outcome measures. Generally, IFN activates multiple signaling pathways, especially JAK. Accordingly, a phase 2 trial published on Lancet proved that baricitinib, a selective JAK1/JAK2 inhibitor, was effective in improving the signs and symptoms of active SLE patients who were inadequately controlled despite standard of care therapy.[50] Furthermore, clinical scientists newly discovered that tofacitinib, a JAK1/JAK3 inhibitor, rapidly alleviated arthritis and partially improved skin rash in patients with SLE, meanwhile brought more patients in clinical remission by sparing steroid.[51] JAK inhibitors have already been used to treat many autoimmune diseases,[52] and whether they will be new players in the field of SLE is keenly awaited [Table 1].
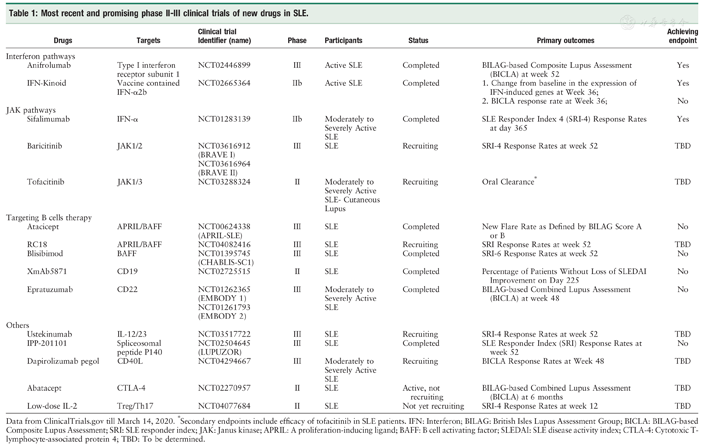
Most recent and promising phase II-III clinical trials of new drugs in SLE.
Most recent and promising phase II-III clinical trials of new drugs in SLE.
| Drugs | Targets | Clinical trial Identifier (name) | Phase | Participants | Status | Primary outcomes | Achieving endpoint | |
|---|---|---|---|---|---|---|---|---|
| Interferon pathways | ||||||||
| Anifrolumab | Type I interferon receptor subunit 1 | NCT02446899 | III | Active SLE | Completed | BILAG-based Composite Lupus Assessment (BICLA) at week 52 | Yes | |
| IFN-Kinoid | Vaccine contained IFN-α2b | NCT02665364 | IIb | Active SLE | Completed | 1. Change from baseline in the expression of IFN-induced genes at Week 36; 2. BICLA response rate at Week 36; | Yes No | |
| JAK pathways | ||||||||
| Sifalimumab | IFN-α | NCT01283139 | IIb | Moderately to Severely Active SLE | Completed | SLE Responder Index 4 (SRI-4) Response Rates at day 365 | Yes | |
| Baricitinib | JAK1/2 | NCT03616912 (BRAVE I) NCT03616964 (BRAVE II) | III | SLE | Recruiting | SRI-4 Response Rates at week 52 | TBD | |
| Tofacitinib | JAK1/3 | NCT03288324 | II | Moderately to Severely Active SLE-Cutaneous Lupus | Recruiting | Oral Clearance* | TBD | |
| Targeting B cells therapy | ||||||||
| Atacicept | APRIL/BAFF | NCT00624338 (APRIL-SLE) | III | SLE | Completed | New Flare Rate as Defined by BILAG Score A or B | No | |
| RC18 | APRIL/BAFF | NCT04082416 | III | SLE | Recruiting | SRI Response Rates at week 52 | TBD | |
| Blisibimod | BAFF | NCT01395745 (CHABLIS-SC1) | III | SLE | Completed | SRI-6 Response Rates at week 52 | No | |
| XmAb5871 | CD19 | NCT02725515 | II | SLE | Completed | Percentage of Patients Without Loss of SLEDAI Improvement on Day 225 | No | |
| Epratuzumab | CD22 | NCT01262365 (EMBODY 1) NCT01261793 (EMBODY 2) | III | Moderately to Severely Active SLE | Completed | BILAG-based Combined Lupus Assessment (BICLA) at week 48 | No | |
| Others | ||||||||
| Ustekinumab | IL-12/23 | NCT03517722 | III | SLE | Recruiting | SRI-4 Response Rates at week 52 | TBD | |
| IPP-201101 | Spliceosomal peptide P140 | NCT02504645 (LUPUZOR) | III | SLE | Completed | SLE Responder Index (SRI) Response Rates at week 52 | No | |
| Dapirolizumab pegol | CD40L | NCT04294667 | III | Moderately to Severely Active SLE | Recruiting | BICLA Response Rates at Week 48 | TBD | |
| Abatacept | CTLA-4 | NCT02270957 | II | SLE | Active, not recruiting | BILAG-based Combined Lupus Assessment (BICLA) at 6 months | TBD | |
| Low-dose IL-2 | Treg/Th17 | NCT04077684 | II | SLE | Not yet recruiting | SRI-4 Response Rates at week 12 | TBD | |
Data from ClinicalTrials.gov till March 14, 2020. *Secondary endpoints include efficacy of tofacitinib in SLE patients. IFN: Interferon; BILAG: British Isles Lupus Assessment Group; BICLA: BILAG-based Composite Lupus Assessment; SRI: SLE responder index; JAK: Janus kinase; APRIL: A proliferation-inducing ligand; BAFF: B cell activating factor; SLEDAI: SLE disease activity index; CTLA-4: Cytotoxic T-lymphocyte-associated protein 4; TBD: To be determined.
Autoreactive B cells are the most important effector cells which are responsible for the perpetuation of the inflammation responses. Therefore, targeting B cells is a very important milestone for SLE treatment.[53] Take belimumab as an example, it is a fully humanized monoclonal antibody designed to specifically target B cells activating factor (BAFF) so as to be able to inhibit the activation of B cells. Similarly, atacicept, a dual inhibitor, can deactivate B cells through binding to both BAFF and a proliferation-inducing ligand (APRIL). A phase IIb study (NCT01972568) reported that SLE sub-populations with high disease activity at baseline and/or serologically active disease had statistically significant improvement in SRI-4 and SRI-6 response rates when they were treated by atacicept compared to placebo.[54] But the efficacy of atacicept in the whole SLE population remains controversial and the safety profiles should be concerned for some severe adverse events which occurred in previous RCTs.[53] Notably, a novel modified dual APRIL/BAFF inhibitor-RCT-18 (also known as telitacicept) was originally developed by a Chinese pharmaceutical company. In July 2019, a key phase II/III trial revealed that SLE patients had a significantly higher SRI-4 response rate in telitacicept group than placebo group at week 48 (79.2% vs. 32.0%, respectively).[55] As such, telitacicept is an exciting candidate that warrants further study in the context of SLE treatment.
Despite the negative findings of rituximab in RCTs in SLE patients for various reasons, anti-CD20 antibody remains a recommended treatment option for patients with refractory and severe disease.[13] Several fully humanized, next generation anti-CD20 monoclonal antibodies, including ocrelizumab, obinutuzumab, and ofatumumab were produced to avoid allergy-like responses. The efficacy and safety of those novel anti-CD20 antibodies in extra-renal SLE as well as LN are under investigation.[56] Anti-CD19 targeting therapy might be an attractive alternative in depleting B cells. XmAb5871, also known as obexelimab, was designed to bind FcγRIIb with its Fc domain and with a humanized Fv region against CD19. To date, the only randomized, double-blinded, placebo-controlled phase II trial of XmAb5871 in SLE is currently reporting the results (NCT02725515). Patients receiving XmAb5871 infusion showed a tendency, however, no statistically significant difference, of higher probability to achieve the primary endpoint than placebo group. Despite the disappointment in many trials and their outcomes, targeting B cells is still one of the most promising therapies in SLE. Virtually how to balance the efficacy and safety of those regimes becomes a big challenge in the field of drug development for SLE.
Immune imbalance between effector and regulatory CD4+ T cells was observed in SLE. Low doses of interleukin-2 (IL-2) can regulate CD4+ T cell subsets and subsequently was used to treat SLE, as described in a previous publication in Nature Medicine.[57] Recently, a randomized, double-blind, placebo-controlled study performed by the team of the Peking University People’s Hospital demonstrated that low-dose IL-2 treatment resulted in a higher SRI-4 response rate with no more adverse events in active SLE patients compared with placebo group.[58] The promising data warrant further multicenter large-scale RCT studies. The preliminary data from a small RCT published in 2019 indicated that omalizumab, a monoclonal antibody against IgE, was associated with improvement in disease activity in SLE patients, with good tolerance.[59] It was gratifying that traditional Chinese medicine, artemisinin may be a potential alternative for SLE treatment. A multi-center phase I RCT showed a significantly more favorable response to artemisinin than placebo in mild/moderate SLE patients. Further investigations of these exciting new agents are extremely encouraged and to be expected. The most recent and promising phase II-III clinical trials of new drugs in SLE from ClinicalTrials.gov are summarized in Table 1.
A number of aforementioned new drugs clearly offer hope for the SLE treatment. It may not take too long to have a range of biological options to treat SLE patients, which matches the choices we have for rheumatoid arthritis patients.
As the number of published articles in the field of SLE increases significantly year by year, it is sometimes helpful to hold on and review what might have passed us by. The current review discussed the highlights of the past year (2019) in clinical and basic researches, mainly focused on the novel insights of pathogenesis, updated recommendations, hot-spot issues on clinical manifestations and prognosis, and most importantly, the therapeutic advances in SLE. As the rapid progress of early detection and management, SLE patients have experienced a great improvement of survival from 1950s to 2000s. But it should be pointed out that the premature mortality gap between SLE patients and general population has never been closed up. Gratifyingly, along with belimumab, an emerging number of novel promising drugs were investigated in clinical trials, and some even showed great potential for SLE with promising data. Nowadays, SLE is being attacked from all sides to gain better insights, and it is for sure that bright future for SLE patients is around the corner.
None.






























