
Hypocretin (HCRT) signaling plays an important role in the pathogenesis of narcolepsy and can be significantly influenced by Chinese herbal therapy. Our previous study showed that xingshentongqiao decoction (XSTQ) is clinically effective for the treatment of narcolepsy. To determine whether XSTQ improves narcolepsy by modulating HCRT signaling, we investigated its effects on SH-SY5Y cell proliferation, apoptosis, and HCRT receptor 1/2 (orexin receptor 1 [OX1R] and orexin receptor 2 [OX2R]) expression. The signaling pathways involved in these processes were also assessed.
The effects of XSTQ on proliferation and apoptosis in SH-SY5Y cells were assessed using cell counting kit-8 and annexin V-fluorescein isothiocyanate assays. OX1R and OX2R expression was assessed by quantitative real-time polymerase chain reaction analysis. Western blotting for mitogen-activated protein kinase (MAPK) pathway activation was performed to further assess the signaling mechanism of XSTQ.
XSTQ reduced the proliferation and induced apoptosis of SH-SY5Y cells. This effect was accompanied by the upregulation of OX1R and OX2R expression and the reduced phosphorylation of extracellular signal-regulated kinase (Erk) 1/2, p38 MAPK and c-Jun N-terminal kinase (JNK).
XSTQ inhibits proliferation and induces apoptosis in SH-SY5Y cells. XSTQ also promotes OX1R and OX2R expression. These effects are associated with the repression of the Erk1/2, p38 MAPK, and JNK signaling pathways. These results define a molecular mechanism for XSTQ in regulating HCRT and MAPK activation, which may explain its ability to treat narcolepsy.

版权归中华医学会所有。
未经授权,不得转载、摘编本刊文章,不得使用本刊的版式设计。
除非特别声明,本刊刊出的所有文章不代表中华医学会和本刊编委会的观点。
Narcolepsy is an obstinate neurological disorder characterized by excessive daytime sleepiness, cataplexy, hypnagogic hallucinations, sleep paralysis, and disturbed nocturnal sleep patterns.[1] Deficiency of the hypocretin (HCRT) system has been confirmed as an underlying cause of most cases of narcolepsy.[2] The orexigenic peptides, HCRT-1 and HCRT-2 (HCRT, also known as orexin (OX)-A and OX-B), are proteolytically cleaved from a common precursor, prepro-OX, and share 46% amino acid sequence identity.[3] These peptides also play a role in the central regulation of feeding and energy homeostasis.[3,4] The physiological effects of OX-A and OX-B are mediated by two G-protein coupled receptors, OX receptor 1 (OX1R) and OX2R, also known as HCRT receptor 1 (HCRTr-1) and HCRTr-2.[5] Knockout of OX-A, OX-B, OX1R, or OX2R produces a narcoleptic mouse model.[6] Furthermore, natural mutations of the OX2R are directly linked to the pathology of narcolepsy.[7]
The intracellular signaling pathways mediating the physiologic function of OXs and their receptors have been under intense investigation.[8] Recent studies have demonstrated that OX-A induces extracellular signal-regulated kinase (Erk1/2) activation via multiple signaling mechanisms in the over-expressing OX1R Chinese hamster ovary cells in vitro.[9] Furthermore, OX-A-induced Erk1/2 activation appears to be protective, whereas p38 mitogen-activated protein kinase (MAPK) activation induces cell death.[10] The activation of Erk1/2, p38 MAPK, and c-Jun N-terminal kinase (JNK) are important in cell proliferation and apoptosis.[11,12,13,14,15,16,17,18] Nevertheless, the precise roles of the signaling pathways of HCRT in narcolepsy remain unclear.
Our previous studies showed that spleen deficiency and the stagnated heat in the liver were the most common syndromes in narcolepsy.[19,20] Xingshentongqiao decoction (XSTQ), a significantly effective traditional Chinese medicine preparation for narcolepsy, is intended to correct the basic pathogenesis of the imbalance of yin and yang for narcolepsy, and this preparation uses herbs that have been proven to have obvious effects targeting the signaling pathways of HCRT.[21] This study aimed to explore the molecular mechanism of XSTQ in targeting the HCRT signaling pathway for narcolepsy.
The herbs of XSTQ were obtained from the Pharmacy of Traditional Medicine of Peking University People’s Hospital (Beijing, China). The human SH-SY5Y cell line was obtained from the cell resource center of Peking Union Medical College. Cell counting kit-8 (CCK-8) was purchased from Dojindo Laboratories (Kumamoto, Japan). Annexin V-fluorescein isothiocyanate (FITC) apoptosis detection kits were obtained from BD Biosciences (San Jose, California, USA). RNeasy Mini kits were purchased from Qiagen (Valencia, CA, USA). The SuperScript™ III First-Strand Synthesis System was purchased from Invitrogen Corporation (Carlsbad, CA). 2.5 × SYBR Green Real Master Mix was purchased from TIANGEN Biotech Co Ltd (Beijing, China). Nitrocellulose filter membranes were purchased from Whatman (Maidstone, UK), and SuperSignal® West Pico chemiluminescent substrate was purchased from Pierce Biotechnology (Rockford, USA). Antibodies to p-Erk1/2, Erk, p-p38 MAPK, p38 MAPK, p-JNK, JNK and anti-rabbit immunoglobulin G (IgG), horseradish peroxidase (HRP)-linked antibody were purchased from Cell Signaling Technology Inc (Danvers, MA, USA).
Xingshentongqiao decoction was made by the water decocting method and then filtered and degermed using 0.22 μm filters. The drug concentration was adjusted to 200 μg/μL with sterilized double distilled water.[22]
SH-SY5Y cells were grown in Dulbecco’s modified eagle medium: Nutrient mixture F-12 (1:1) medium (DMEM/F12) (Gibco, Invitrogen, Carlsbad, California, USA) with 10% fetal bovine serum, 100 units/mL penicillin, and 100 μg/mL streptomycin (Gibco, Invitrogen) at 37°C under 5% CO2 and 95% ambient air.
Dose and time dependent cell viability was monitored using the CCK-8 assay, a sensitive nonradioactive colorimetric assay used to determine the number of viable cells in proliferation and cytotoxicity assays. SH-SY5Y cells were cultured in 96-well plates containing growth culture medium at a density of 2 × 103 cells/well. The cells were divided into seven groups: Control group and XSTQ treatment groups (100, 200, 400, 600, 800, and 1000 μg/ml XSTQ). Each group was further treated for 0, 24, 48, 72, or 96 hours separately as described, after which CCK-8 solution was added to the cell culture medium to a final concentration of 10 μl/100 μl, and the cells were incubated for another 3 hours at 37°C. Absorbance was measured at 450 nm to determine cell viability as a percentage using a microplate reader (iMark; Bio-Rad, Hercules, CA, USA).
Cells were seeded by placing 1 × 106/mL of cell culture in 10 cm cell culture dishes and maintained for 24 h at 37°C. XSTQ (0, 10, 100 or 1000 μg/ml) was directly added to the dishes, which were incubated for an additional 24 hours. The Annexin V-FITC apoptosis detection kit was used to measure apoptosis levels according to the manufacturer’s instructions.
Total ribonucleic acid (RNA) was isolated from SH-SY5Y cells using RNeasy Mini kits according to the manufacturer’s instructions. The purity and concentration of RNA were measured using a Gene Quant pro device (Nanovue Spectrophotometer, GE Healthcare, London, UK). Complementary deoxyribonucleic acid (cDNA) was synthesized with 2.5 μg total RNAusing the SuperScript™ III First-Strand Synthesis System according to standard protocol. Quantitative real-time polymerase chain reaction (qRT-PCR) was performed using a detector (Bio-Rad, Munich, Germany). The PCR solution contained specific primers (0.5 μl each/10 μmol/L), 4.0 μl 2.5 × SYBR Green Real Master Mix, and 0.25 μL cDNA with a final volume of 10 μL. The following primers were used: Human OX1R sense: 5’-CAG CCT ATG TGG CTG TGT TCG T-3’, antisense: 5’-AGT TGG TGA CTG TCC TCA TGT GG-3’); human OX2R sense: 5’-GTG TTC GTC GTG GCT CTC ATT G-3’, antisense: 5’-CAG GTG ATG GTC ACG AGC ACA T-3’) and human β-actin sense: 5’-CTG GAA CGG TGA AGG TGA CA-3’, antisense: 5’-AAG GGA CTT CCT GTAACA ATG CA-3’. The reaction conditions for amplifying DNA were 94°C for 2 minutes, followed by 45 cycles of 94°C for 20 seconds, 62°C for 20 seconds, and 72°C for 30 seconds. The messenger RNA (mRNA) expression was normalized to the expression level of β-actin and was calculated using the following equation: Fold change = 2−ΔΔCT.
After 24 hours serum deprivation, SH-SY5Y cells were treated with XSTQ at indicated concentrations for the times indicated. Then they were washed in phosphate buffered saline twice and homogenized in an ice cold lysis buffer containing 1% triton X-100, 20 mmol/L Tris-HCl (pH 7.5), 150 mmol/L NaCl, 1 mmol/L Hepes, 10% glycerol, 1 mmol/L NaF, 1 mmol/L Na3VO4, and 1% protease inhibitors (Amresco Corporation, USA). The homogenates were centrifuged at 12,000 r/min for 20 minutes at 4°C. An aliquot of the supernatant was removed for protein assay, and the remaining supernatant was added to an equal volume of sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) sample buffer. Samples (125 μg protein/lane) were subjected to 10% SDS-PAGE and transferred electrophoretically onto nitrocellulose membranes using a Mini PROTEAN 4 cell device (Mini-PROTEAN 4, Bio-Rad, Hercules, CA, USA) and Mini Trans-Blot electrophoretic transfer cell (Mini-PROTEAN 4, Bio-Rad) at 100 V for 2 hours. The membranes were incubated with 5% nonfat dried milk for 2 h at room temperature to block nonspecific binding and then incubated with rabbit anti-p-Erk1/2 (1:1000), anti-p-p38 MAPK (Thr180/Tyr182) (1:500), anti-p-JNK (Thr183/Tyr185) (1:1000), anti-Erk1/2 (1:1000), anti-p38 MAPK (1:1000) or anti-JNK (1:1000) antibodies at 4°C overnight. The membranes were washed three times for 10 minutes each in tris-buffered saline with Tween 20 (TBST) (20 mmol/L Tris-HCl, 137 mmol/L NaCl, 0.05% Tween-20) and then incubated for 1 hour with anti-rabbit IgG, HRP-linked antibody (1:2000). The membranes were washed three additional times for 10 minutes each in TBST, and the signals were detected using the SuperSignal® West Pico chemiluminescent substrate. The bands were captured, and their optical density was analyzed with software Image J. Each experiment was repeated three times, and the mean value was presented as the experimental result.
The statistical analysis was performed using software Statistical Package for the Social Sciences (SPSS) version 18.0 (SPSS, Chicago, IL, USA). Data are expressed as mean ± standard deviation (SD). Statistical analysis was performed using one-way analysis of variance or the Student’s t-test. P <0.05 was considered as statistically significant.
To determine whether XSTQ affects cell proliferation, we treated SH-SY5Y cells with a range of doses and times of XSTQ. SH-SY5Y proliferation was inhibited in a dose-dependent manner after 72 hours and 96 hours treatment with XSTQ at concentrations of 400–1000 μg/ml [Table 1, Figure 1]. These results suggest that XSTQ inhibits proliferation.
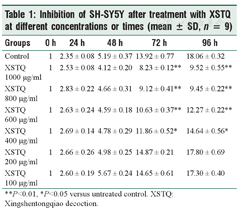
Inhibition of SH-SY5Y after treatment with XSTQ at different concentrations or times (mean ± SD, n = 9)
Inhibition of SH-SY5Y after treatment with XSTQ at different concentrations or times (mean ± SD, n = 9)
| Groups | 0 h | 24 h | 48 h | 72 h | 96 h |
| Control | 1 | 2.35 ± 0.08 | 5.19 ± 0.37 | 13.92 ± 0.77 | 18.06 ± 0.32 |
| XSTQ 1000 μg/ml | 1 | 2.53 ± 0.08 | 4.12 ± 0.20 | 8.23 ± 0.12** | 9.52 ± 0.55** |
| XSTQ 800 μg/ml | 1 | 2.83 ± 0.22 | 4.66 ± 0.31 | 9.12 ± 0.41** | 9.45 ± 0.22** |
| XSTQ 600 μg/ml | 1 | 2.63 ± 0.24 | 4.59 ± 0.18 | 10.63 ± 0.37** | 12.27 ± 0.22** |
| XSTQ 400 μg/ml | 1 | 2.69 ± 0.14 | 4.78 ± 0.29 | 11.86 ± 0.52* | 14.64 ± 0.56* |
| XSTQ 200 μg/ml | 1 | 2.66 ± 0.26 | 4.98 ± 0.25 | 14.87 ± 0.21 | 17.80 ± 0.69 |
| XSTQ 100 μg/ml | 1 | 2.60 ± 0.19 | 5.67 ± 0.24 | 14.65 ± 0.61 | 17.30 ± 0.40 |
**P<0.01, *P<0.05 versus untreated control. XSTQ: Xingshentongqiao decoction.
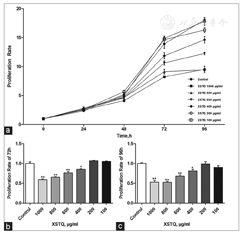

To determine whether XSTQ also affects cell apoptosis, we performed Annexin V-FITC staining assays after 24 h treatment of SH-SY5Y cells with a range of doses of XSTQ (0, 10, 100 and 1000 μg/mL). The apoptotic rates were 3.65% ± 0.12%, 3.05% ± 0.18%, 3.23% ± 0.22% and 4.72% ± 0.16%, respectively. Statistical analysis suggests that the apoptotic rate of cells treated with 1000 μg/ml XSTQ was higher than that of the control group [Figure 2]. These findings suggest that XSTQ promotes apoptosis.
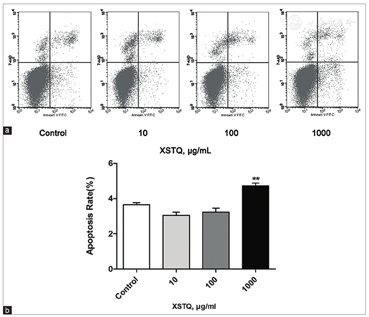

Deficiency of the HCRT system has been confirmed as an underlying cause of most cases of narcolepsy.[2] To test whether XSTQ counteracts narcolepsy by modulating components within the HCRT signaling cascade, we assessed levels of expression of the HCRT receptors OX1R and OX2R. mRNA was collected from SH-SY5Y cells treated with XSTQ (1000 μg/ml) for 0, 24, 48, and 72 hours, and transcript levels were quantified by qRT-PCR. XSTQ induced an obvious increase in OX1R and OX2R mRNA expression, reaching a maximum at 24–48 hours [Figure 3]. These results suggest that XSTQ may counteract narcolepsy by enhancing the expression of HCRT signaling receptors.
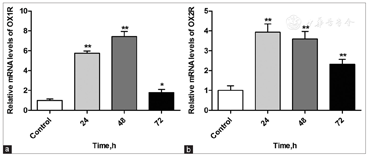

To identify HCRT-related signaling pathways that may be affected by XSTQ, we examined the effects on Erk1/2, p38 MAPK and JNK activation. Increasing concentrations of XSTQ were added to SH-SY5Y cells, and levels of phosphorylated and total protein were assessed by western blotting. XSTQ significantly downregulated the phosphorylation of Erk1/2, p38 MAPK and JNK at 1000 μg/ml concentration. Furthermore, XSTQ downregulated the phosphorylation of Erk1/2 and JNK at 100 μg/ml and Erk1/2 at 10 μg/ml [Figure 4].
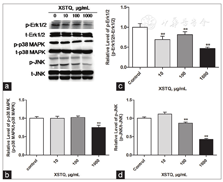

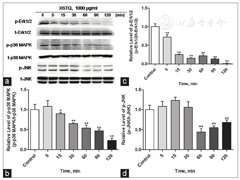
To verify the inhibition of Erk1/2, p38 and JNK activation by XSTQ, we examined the time dependence of the inhibition at 1000 μg/ml XSTQ. XSTQ promoted a time-dependent-downregulation of the phosphorylation of all three proteins compared with the control group [Figure 5]. These results confirm the effects of XSTQ in inhibiting these three MAPK signaling pathways and provide an additional mechanism to explain the XSTQ-dependent effects on proliferation and cell death.

Orexins, also known as HCRTs, are neuropeptides produced in neurons of the lateral and dorsomedial hypothalamus and perifornical area, which project widely throughout the brain. The absence of OXs in rodents and humans leads to narcolepsy.[23] Blockade of OX1R and OX2R antagonism elicits a dysregulation of rapid eye movement (REM) sleep by shifting the balance in favor of REM sleep at the expense of nonREM sleep and may increase the risk of adverse events.[24] Moreover, knockout of either OX1R or OX2R produces a narcoleptic rat model.[6] In this study, we found that XSTQ upregulated OX1R and OX2R expression in SH-SY5Y cells in vitro. The deficiency of OX1R or OX2R is one of the critical factors contributing to the pathogenesis of narcolepsy.[25] In addition, HCRT (OX) deficiency (<40 pg/ml) is highly associated with clinical narcolepsy and cataplexy (89.5%), and the HCRT content in the hypothalamus is decreased significantly in autopsies of narcoleptic patients.[26] These observations indicate that upregulation of OX1R and OX2R expression may be one of the critical molecular mechanisms of XSTQ in protecting against narcolepsy.
We also examined the ability of XSTQ to affect cell proliferation and apoptosis as an additional potential mechanism of protection against narcolepsy. Narcolepsy is accompanied by the proliferation of histamine neurons, which can cause classic symptoms of narcolepsy, such as cataplexy and hypnagogic hallucinations.[27,28] In addition, an increase of cholinergic receptors is one of the most significant features of narcolepsy, which might comprise an adaptive response to the malfunction of the secretion of acetylcholine and dopamine.[29] Previous studies show that retinoic acid-differentiated SH-SY5Y cells have a DAergic-like phenotype, and play a critical role in the secretion of histamine and dopamine.[30,31] In the current study, incubation of SH-SY5Y cells with 400–1000 μg/ml XSTQ was shown to significantly inhibit cell proliferation after 72 hours and 96 hours, as determined by CCK-8 assay. The effect of XSTQ on SH-SY5Y cell apoptosis at 1000 μg/ml was also verified by Annexin V-FITC apoptosis detection. Our study indicates that XSTQ can inhibit SH-SY5Y cell proliferation and promote SH-SY5Y cell apoptosis in vitro. These results are consistent with the possibility that XSTQ may regulate abnormal proliferation and apoptosis of histamine neurons in narcolepsy.
To gain further insight into the molecular mechanisms by which XSTQ mediates proliferation and apoptosis in SH-SY5Y cells, we examined intracellular signaling pathways. Treatment of SH-SY5Y cells with various concentrations of XSTQ significantly decreased phosphorylation of Erk1/2, p38 MAPK and JNK. Phosphorylation of Erk1/2, p38 MAPK and JNK are involved in cell proliferation and apoptosis.[11,12,13,14,15,16,17,18] Furthermore, tissue kallikrein promotes SH-SY5Y cell proliferation through the Erk1/2/MAPK signaling pathway,[32] and effects of propofol on proliferation and anti-apoptosis of SH-SY5Y cells are mediated via Erk1/2/MAPK signaling.[33] Sphingomyelinase metabolites also control survival and apoptotic death in SH-SY5Y cells through p38 MAPK signaling pathways,[34] while JNK can mediates either cell death and proliferation of SH-SY5Y cells.[35] Therefore, the observation that XSTQ reduces the phosphorylation of these signaling proteins provides a mechanism for this preparation in the suppression of proliferation and activation of SH-SY5Y cell death.
Xingshentongqiao decoction upregulates OX1R and OX2R expression in SH-SY5Y cells in vitro, which could play a critical role in the activation of HCRT signaling pathways in narcolepsy. Furthermore, XSTQ inhibits proliferation and induces apoptosis in SH-SY5Y cells, which may be mediated through its effects in repressing the Erk1/2, p38 MAPK and JNK pathways. This study expands our knowledge of the molecular mechanisms of XSTQ on the HCRT signaling pathways in narcolepsy and suggests that HCRT signaling may provide a target for the beneficial effects of XSTQ in the treatment of narcolepsy with syndromes of spleen deficiency and the stagnated heat in the liver.






























