
Many patients develop a variety of bowel dysfunction after sphincter preserving surgeries (SPS) for rectal cancer. The bowel dysfunction usually manifests in the form of low anterior resection syndrome (LARS), which has a negative impact on the patients' quality of life. This study reviewed the LARS after SPS, its mechanism, risk factors, diagnosis, prevention, and treatment based on previously published studies. Adequate history taking, physical examination of the patients, using validated questionnaires and other diagnostic tools are important for assessment of LARS severity. Treatment of LARS should be tailored to each patient. Multimodal therapy is usually needed for patients with major LARS with acceptable results. The treatment includes conservative management in the form of medical, pelvic floor rehabilitation and transanal irrigation and invasive procedures including neuromodulation. If this treatment failed, fecal diversion may be needed. In conclusion, Initial meticulous dissection with preservation of nerves and creation of a neorectal reservoir during anastomosis and proper Kegel exercise of the anal sphincter can minimize the occurrence of LARS. Pre-treatment counseling is an essential step for patients who have risk factors for developing LARS.

Copyright © 2020 The Chinese Medical Association, produced by Wolters Kluwer, Inc. under the CC-BY-NC-ND license. This is an open access article distributed under the terms of the Creative Commons Attribution-Non Commercial-No Derivatives License 4.0 (CCBY-NC-ND), where it is permissible to download and share the work provided it is properly cited. The work cannot be changed in any way or used commercially without permission from the journal.
Colorectal cancer is the third most commonly diagnosed cancer in the world.[1] During the last two decades, significant evolution in the staging and treatment of rectal cancer, together with advances in the pre-operative neoadjuvant chemoradiotherapy had improved the survival and decreased the local recurrence rate.[2,3] Recently, there is a shifting paradigm in the treatment of low rectal cancer from abdominoperineal resection (APR) into sphincter preserving surgeries (SPS), due to advances in surgical techniques including minimally invasive surgery and pre-operative chemoradiotherapy.[4,5] The indication for SPS was expanded to very low-lying tumors within 1 to 3 cm from the anal verge. With acceptable oncologic outcomes, the ultralow anterior resection (ULAR) with coloanal anastomosis[6] and the intersphincteric resection (ISR) have been administered.[7] In spite of this advance, still many patients suffer from post-operative bowel dysfunction not only in the form of stool frequency, urgency, evacuation problems which is named low anterior resection syndrome (LARS), but also variant degrees of fecal incontinence (FI) that have negative impact on patients’ quality of life (QoL).[8,9] There is variability in the literature’s results reporting the LARS incidence. Some studies reported its incidence ranging from 19% to 52%.[10] In a recent meta-analysis by Croese et al,[11] the estimated prevalence of LARS was 41%.
The mechanism of LARS is thought to be due to decreasing volume of the rectal reservoir together with hypermobility of the neorectum because of denervation of the colon during surgery and lateral mobilization which can lead to the urgency and frequency manifestations. Additionally, some degrees of damage to the internal sphincter during anastomosis creation or ISR of very low lying rectal cancer also contribute to the symptoms of FI.[12] In spite of the available treatment options, till now there is no standard treatment for LARS.[13] Some authors pointed out the effect of physical exercise on improving the bowel function especially in females who survived from rectal cancer and its impact on the health-related QoL.[14]
The purpose of this review was to focus on the bowel dysfunction after SPS, evaluation of the pathophysiology, risk factors, proper treatment modalities of LARS, and the effect of bowel dysfunction on the QoL of the patients.
LARS is usually caused by a combination of three factors: colonic dysmotility, neorectal reservoir dysfunction, and anal sphincter dysfunction.
Increased proximal colonic motility coupled with a lack of distal rectal inhibition due to denervation of the sigmoid or descending colon during surgery, leading to increased colonic motility.[15] In comparison to normal persons, patients with LARS have a shorter colonic transit time and a greater increase in neorectal pressure after a meal. Furthermore, lack of the negative feedback signals from the rectum and rectosigmoid junction which normally represent distal control center for regulation of bowel transit to oppose the increased proximal colonic motility, leaves the bowel activity without a brake and exacerbates the LARS symptoms.[16]
It refers to any denervation and reduction in functional capacity of the neorectum that cause LARS symptoms. The neorectum may be denervated by surgery or pelvic radiotherapy and hence is hyposensitive to mechanical and thermal stimuli. There is evidence that the risk of LARS is greater after total mesorectal excision (TME) in comparison to partial mesorectal excision (PME) and after neoadjuvant therapy. Besides denervation, reduction in neorectal capacity and compliance, leads to more dysfunction. After rectal surgery, smaller volumes of fecal material are sufficient to induce contraction or spasm of the neorectum which can cause symptoms such as urgency, soiling, or multiple evacuations.[17]
It may occur as a complication of rectal surgery or preoperative pelvic radiotherapy. Farouk et al[18] reported in their study that, up to 18% of patients who underwent stapled anastomosis after low anterior resection (LAR) suffered from long-term internal anal sphincter (IAS) injury. Moreover, ISR can affect sphincter functions leading to variable degrees of FI, urgency, and soiling especially due to excision of anal transition zone.[19] On long-term follow-up, scarring of IAS or fibrosis of the pudendal nerve has been reported after radiotherapy leading to decrease in the mean resting and squeeze anal pressure.[19] Rectoanal inhibitory reflex is affected by surgery or radiotherapy because of autonomic nerve injury, which may result in reduction of anal resting pressure and fecal soiling.[20]
LARS can occur after LAR for rectal cancers and after anterior resection for high tumors but with low frequency. Understanding the possible risk factors of LARS may help in surgical decision making and post-operative anorectal rehabilitation programs for the patients.[10]
Risk factors for developing LARS are low colorectal or coloanal anastomosis, end-to-end anastomosis, anastomotic leakage (AL), and adjuvant radiotherapy, and the level of anastomosis is the most important factor.[21] Other risk factors that have been reported to be associated with LARS are female sex,[22] presence of stoma, and post-operative complications.[23] Cheong et al[24] addressed the risk factors for bowel dysfunctions after LAR and ULAR and found, old age, male sex, adjuvant chemoradiation therapy, and ULAR were all risk factors. Another randomized trial showed almost two-fold higher LARS prevalence in patients who received radiotherapy and surgery than in patients who underwent surgery alone for rectal cancer.[25] Alavi et al[26] in their study on rectal cancer patients, noticed that low tumor location, radiation therapy, temporary ostomy, and smoking history were associated with long-term bowel dysfunction following an anastomosis. In another study addressing the bowel functions of rectal cancer patients, Park et al,[27] reported that pre-operative chemoradiation, short time interval from restoration of bowel continuity, hand-sewn anastomosis, and ileostomy were significantly associated with poor bowel function or major FI. Moreover, patients undergoing TME are at greater risk of developing LARS than those having PME.[28] Badic et al,[29] reported that FI was significantly correlated with neoadjuvant chemoradiotherapy, low rectal resection, and tumor distance from anal verge but not with stage, gender, or obesity. From the above reports, we can find the early and delayed impact of neoadjuvant radiation therapy on the bowel function. In a study by Pollack et al,[30] assessing the long term effect of short-course radiotherapy on anorectal function using questionnaire, anal manometry, and endorectal ultrasound (ERUS), they noted that irradiated patients had significantly more symptoms of FI, soiling and more bowel movements per week, lower anal resting and squeeze pressures (SPs), more scarring of the anal sphincters by using endoanal ultrasound. The same results were noted by Peeters et al[31] in their study, in which they found that, irradiated patients reported increased rates of FI, pad wearing, anal blood, and mucus loss. Furthermore, their overall satisfaction with bowel function was significantly lower than patients who underwent TME alone. Quezada-Diaz et al[32] investigated the effect of neoadjuvant systemic chemotherapy with or without chemoradiotherapy on bowel function after TME for rectal cancer patients using Memorial Sloan-Kettering Cancer Center Bowel Function Instrument (MSKCC BFI), and noted that exposure to radiotherapy only was correlated with worse bowel function which was not observed after exposure to neoadjuvant chemotherapy. So, neoadjuvant chemotherapy, may not affect the bowel function.
AL negatively affects the bowel function and the patient’s QoL after SPS for rectal cancer, and the mechanism is mostly due to scarring of the neorectal reservoir and reduction of its volume.[33] Ashburn et al[34] found in their study that patients with AL suffered from more frequent bowel movements at daytime and night-time than patients without AL at 1 year of follow up after rectal resection. AL patients also had worse control of solid stool, a greater need for pad wearing and bad QoL. Nesbakken et al,[35] reported that AL correlated with a reduction in maximum tolerated volume and difficult evacuation. Urgency and FI were slightly worse after AL, but without significance. Bittorf et al[36] in their retrospective study, on 22 AL/150 patients, urgency and maximum tolerated volume were lower in the AL group. However, there was no significant difference in bowel function and patient satisfaction between both groups.
One important point that should be addressed is investigating whether the TaTME technique does more harm to the bowel function than conventional abdominal TME. This is because the TaTME anastomosis is closer to the anal sphincter than abdominal TME, prolonged dilatation of anal canal by air insufflation system in TaTME may affect the anal sphincter and sometimes the associated damage to the pelvic innervation of levator ani muscle especially with more radical resection in lower pelvis.[37] Koedam et al[37] in their study focused on functional outcomes after TaTME on 30 patients and noticed deterioration of bowel function at 1 month follow up, but at 6 months the bowel function and QoL scores improved and only 30% of patients still have major LARS score. Whereas Atallah et al,[38] through a telephone survey, assessed 20 patients who were examined 2 months after ileostomy closure, they found most patients had less than one bowel movement/day, and only one patient suffered from FI and poor QoL even after 1 year follow up. Elmore et al,[39] followed up six patients using Wexner score after surgery, the mean Wexner score slightly worsened from zero to three at 6 months post-operatively and after 4 months from stoma closure. Hence, waiting for results of the COLOR III trial, a randomized clinical trial comparing laparoscopic TME vs. TaTME, will show enough data about the functional results, QoL, after mid and low restorative rectal cancer surgery.[40] Some of the aforementioned studies are summarized in Table 1.
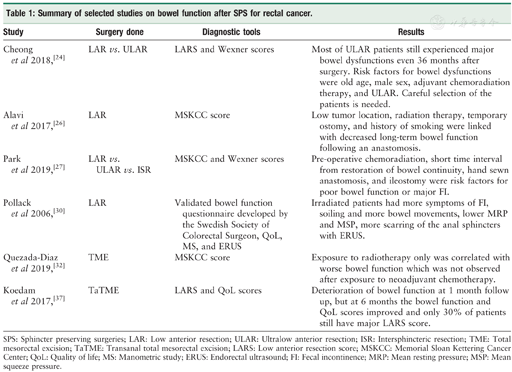
Summary of selected studies on bowel function after SPS for rectal cancer.
Summary of selected studies on bowel function after SPS for rectal cancer.
| Study | Surgery done | Diagnostic tools | Results |
|---|---|---|---|
| Cheong et al 2018,[24] | LAR vs. ULAR | LARS and Wexner scores | Most of ULAR patients still experienced major bowel dysfunctions even 36 months after surgery. Risk factors for bowel dysfunctions were old age, male sex, adjuvant chemoradiation therapy, and ULAR. Careful selection of the patients is needed. |
| Alavi et al 2017,[26] | LAR | MSKCC score | Low tumor location, radiation therapy, temporary ostomy, and history of smoking were linked with decreased long-term bowel function following an anastomosis. |
| Park et al 2019,[27] | LAR vs. ULAR vs. ISR | MSKCC and Wexner scores | Pre-operative chemoradiation, short time interval from restoration of bowel continuity, hand sewn anastomosis, and ileostomy were risk factors for poor bowel function or major FI. |
| Pollack et al 2006,[30] | LAR | Validated bowel function questionnaire developed by the Swedish Society of Colorectal Surgeon, QoL, MS, and ERUS | Irradiated patients had more symptoms of FI, soiling and more bowel movements, lower MRP and MSP, more scarring of the anal sphincters with ERUS. |
| Quezada-Diaz et al 2019,[32] | TME | MSKCC score | Exposure to radiotherapy only was correlated with worse bowel function which was not observed after exposure to neoadjuvant chemotherapy. |
| Koedam et al 2017,[37] | TaTME | LARS and QoL scores | Deterioration of bowel function at 1 month follow up, but at 6 months the bowel function and QoL scores improved and only 30% of patients still have major LARS score. |
SPS: Sphincter preserving surgeries; LAR: Low anterior resection; ULAR: Ultralow anterior resection; ISR: Intersphincteric resection; TME: Total mesorectal excision; TaTME: Transanal total mesorectal excision; LARS: Low anterior resection score; MSKCC: Memorial Sloan Kettering Cancer Center; QoL: Quality of life; MS: Manometric study; ERUS: Endorectal ultrasound; FI: Fecal incontinence; MRP: Mean resting pressure; MSP: Mean squeeze pressure.
There is a close association between bowel function and QoL, as regard to global health status and social functioning. Emmertsen et al found strong associations between all functional QoL scales and major LARS at 1 year.[22] In other words, LARS has a negative impact on the patient’s QoL. Vironen et al,[41] showed that FI and urgency had a significant effect on social functioning, whereas urgency alone negatively affected mental and general health perception. These results also were emphasized by Pucciarelli et al[42] in their study on a group of patients undergoing LAR with pre-operative neoadjuvant chemoradiotherapy, and they found strong relationship between urgency and physical and social functioning.
In a study conducted by Silva et al,[43] 125 patients diagnosed with mid and low rectal adenocarcinoma, 83 patients underwent LAR with low colorectal or coloanal anastomosis, while 42 patients were treated with APR and terminal colostomy. Upon assessment the QoL scores in both groups, the patients who underwent APR had significantly better function and symptom scores reflecting better QoL.
In contrast, Konanz et al[44] assessed the QoL in three matched groups of rectal cancer patients who underwent APR, LAR, and ISR and they noticed no difference in the QoL after SPS compared to APR. However, the ISR group suffered from more FI than LAR group. While other studies, pointed out that the QoL after ISR is not better than QoL after APR because of the incontinence state which hinders the patient from doing daily activity and leads to social isolation.[43]
LARS should be suspected in patients who develop one or more bowel symptoms after undergoing SPS. The diagnosis is confirmed when the symptoms persist more than 1 month after surgery after exclusion of any alternative etiology. It is essential to assess the anorectal function from patient’s perspective because there is misunderstanding of LARS symptoms and its effect on the QoL among rectal cancer experts. Thus, there is a need for questionnaires. Many questionnaires have been proposed for assessing bowel function. The most suitable tools for assessing the bowel function after rectal cancer surgery are the LARS score and the MSKCC BFI.[45] The diagnostic tools of LARS are shown in Figure 1.
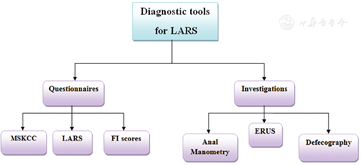

Numerous instruments have been utilized for evaluating the degrees of FI, including the Cleveland Clinic Florida/Wexner fecal incontinence score,[46] the fecal incontinence severity index, [47] and the St. Mark incontinence score.[48] Every one of the three instruments has been demonstrated to correspond with the patient’s subjective perception of bowel control.[46,49] They adopt a similar strategy of surveying the frequency of incontinence episodes. The Wexner score is the most widely utilized tool to date. It examines three types of FI (solid, liquid, and gas) and their consequences (pad wearing and lifestyle alteration). For each item, the five frequency options range from never (score 0) through to always (meaning at least once per day; score 4). The total score is the sum of the item scores that range from 0 (perfect continence) to 20 (complete incontinence).
MSKCC is the first questionnaire designed for evaluating bowel function after SPS for rectal cancer.[50] It has been translated from English into Italian version, and has also been validated.[51] It comprises of 18 questions about the variety of LARS issues over the past 4 weeks. For each question, there are five answer options range from never to always, except for one question asking about the number of bowel movements per day. Response can be summarized into three sub-scales (frequency, six items; diet, four items; and urgency/soilage, four items) by adding the scores of the items in the sub-scale. There are also four single items that do not belong to a sub-scale which include incomplete evacuation, clustering (having another bowel movement within 15 min of the last movement), knowing the difference between needing to pass gas and a bowel movement, and incontinence for flatus. Finally, the total score is calculated by summing the global score and the score for the four single items with a maximum score of 90. A higher score represents better bowel function.[50] The main strengths of the MSKCC BFI are the detailed, comprehensive and thorough evaluation of bowel dysfunction after TME. Additionally, the sub-scales facilitate separate interpretation of different aspects of LARS when desired as well as correlation with some QoL instruments. However, the MSKCC score’s length (18 questions) and scoring (which involves recoding, three sub-scale scores, a global score, and a total score) may limit its usage.
LARS score like the MSKCC BFI, is a questionnaire for assessing bowel function after SPS with or without radiotherapy for rectal cancer. The LARS score was developed from a large cohort of 961 Danish patients, who received LAR for rectal cancer.[52] It includes five questions, incontinence for flatus, incontinence for liquid stool, frequency (number of daily bowel movements), clustering (having to open bowels again within 1 h of the last opening), and urgency. The LARS score does not use a specific recall period. In its initial validation, the ability of the LARS score to reflect the impact of bowel dysfunction on QoL was proven,[52] and so through the association with many of QoL scales.[22,53] The LARS score severity categories (no, minor, and major LARS) can facilitate identification of patients who need treatment. Those with major LARS, report significantly worse QoL than those with No/minor LARS.[22,52,53] In addition to the original Danish version, the LARS score has been translated into several languages (English, Dutch, Swedish, Spanish, and German),[54] and hence has the capacity for widespread use. The greatest advantages of the LARS score lie in its conciseness and ability to show impact on QoL. It is widely used because of the easy scoring and clinical meaningful severity categories.
But here, we have to raise a question: Which questionnaire is the best for assessment of bowel function? For comprehensive and detailed evaluation of LARS, the MSKCC score would be the questionnaire of choice. For rapid screening or assessment of LARS, the LARS score is the ideal for that. Both instruments are valid and reliable to detect clinically relevant differences. Overall, the MSKCC BFI has a broader scope, covering not only LARS symptoms, but also their consequences. On the other hand, the LARS score is more practical, and can correlate with QoL scores.[55]
As a result of the SPS, especially ISR, and neoadjuvant radiotherapy, some defects or scarring in the anal sphincter can be visualized by using the ERUS with an accuracy of over 98%.[56]
It is one of the standard tools for evaluation of anorectal dysfunction in LARS. In patients with LARS, they usually have decreased resting anal sphincter pressure, SP, and low rectal compliance. However, SP sometimes may be normal in patients with LARS.[57] Moreover, if colonic manometry is done in LARS patients, it will often reveal an increase in high-amplitude propagated contractions.
Defecography can be helpful in demonstrating anorectal functional disorders in LARS. Mostly, it shows widened anorectal angle, reduced rectal evacuation and a low volume of the neorectum.[19] MR defecography could reveal detailed information about the anorectal angle, the pelvic floor descent, and anal canal opening.[58]
LARS can be treated with medications, transanal irrigation (TAI), pelvic floor rehabilitation, neurostimulation, or surgery. The choice of treatment is based on the severity, and duration of symptoms.[59] Studies on LARS management are limited, and prospective randomized controlled trials are needed for evidence-based conclusions. However, it is clear that a multimodal approach could represent the best management option for LARS patients rather than a single treatment.[59] The treatment strategies are shown in Figure 2.
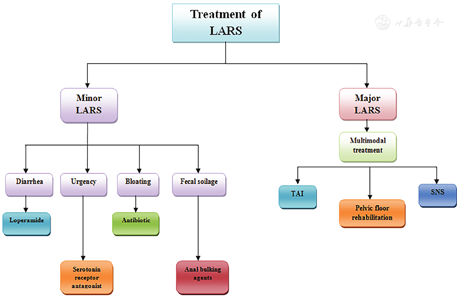

In patients with minor LARS, who are suffering from diarrhea only, can be managed with anti-diarrheal agents like loperamide that has been evaluated in randomized trials in patients with irritable bowel syndrome (IBS). In IBS patients, loperamide improves the stool consistency and decreases the number of bowel movements per day.[60] Moreover, Loperamide significantly decreased stool frequency, modified some aspects of pouch contraction, and improved anal sphincter function in patients who have undergone a restorative proctocolectomy with ileoanal pouch.[61]
Some patients with LARS have an exaggerated postprandial response in the neorectum of urgency and/or incontinence. Serotonin receptor antagonist (ramosetron) plays an important role in control. In a study by Itagaki et al,[62] the authors found that oral serotonin receptor antagonist (5 μg of ramosetron hydrochloride, once daily after breakfast) intake resulted in significant improvement in the urgency grade, incontinence score, and the number of toilet visits per day. The results are better if the therapy is given from the first 6 months post-operatively.
Patients whose main symptoms are excessive flatulence, or abdominal bloating which can be caused by small intestinal bacterial overgrowth transmitted from the colon causing abnormal fermentation of food, can be treated with antibiotics such as rifaximin or neomycin.[63]
It is thought that injection of anal bulking agents like (dextranomer stabilized in hyaluronic acid) may enhance resting anal pressures and thereby improve fecal continence, especially in patients with passive FI or fecal soilage with limited efficacy.[64]
There is no evidence supporting the use of steroids or non-steroidal anti-inflammatory drugs in LARS treatment. Fiber intake improves stool consistency but not any other bowel symptoms, and not well tolerated in LARS patients due to bloating and abdominal pain. Additionally, dietary restrictions, constipating agents, or enemas typically do not add any value to control LARS symptoms and their impact on patient QoL is doubtful.[59] Probiotics have no role in the prevention or treatment of LARS. In one study, the use of a probiotic preparation did not change the post-operative bowel function of patients undergoing loop ileostomy takedown after SPS.[65]
Major LARS has a significant negative impact on patients' QoL. Those patients typically require multimodal therapy rather than medical treatment for individual symptoms. Multimodal therapy comprises TAI at least 3 to 4 times/week plus pelvic floor rehabilitation (biofeedback, pelvic floor muscle training, balloon training, and electrostimulation). This treatment should continue for (6 months–1 year) and then patients should be re-evaluated using the questionnaire again. If symptoms of major LARS improved, persistent minor symptoms can be treated medically. Patients who continue to have major LARS >1 year of multimodal therapy should be treated with neurostimulation (sacral nerve stimulation [SNS]). Patients who do not respond to neurostimulation and have severely compromised QoL more than 2 years from the start of multimodal therapy should be offered a diverting stoma.[59]
It has been reported to be a cheap and effective method for the treatment of FI and frequent bowel movement associated with LARS.[66,67] It includes two forms: low and high volume trans-anal water irrigation. The role of low volume TAI is mechanical washout. Whereas, high volume TAI induces colonic mass movements and improves colonic transit time and FI on regular administration. TAI is more suitable for patients with low rectal capacity, rectal volume, and low anal SP.[59] The efficacy of TAI in treatment of LARS has been investigated in many studies. In a prospective study by Rosen et al,[66] the authors noted significant improvements in the number of defecation times and the incontinence scores. In another systematic review by Christensen and Krogh,[68] TAI showed an improvement in 79% to 100% of patients with LARS after rectal cancer surgery. In a recent randomized control trial by Rosen et al,[69] comparing the effect of TAI vs. supportive therapy in patients who underwent ultralow rectal resection, they found decreasing of the maximum number of defecation per day and per night and a better LARS score after 1 and 3 months among patients who underwent TAI. Wexner score also improved in the TAI group after 3 months. Herein, some questions remain regarding the optimal volume of water needed for TAI, time interval between irrigations, safety, and whether TAI should be considered a lifelong therapy or not.[69] Martellucci et al[70] used a median volume of 450 (300-1000) mL, 3 to 4 times/week with six patients dropped out because of dissatisfaction. In another study, the median volume of water used for TAI was 900 (500–1500) mL/day.[66] In general, this technique is safe and the risk of rectal perforation is very low as reported in some studies (0.002%).[71] It is still unclear, for how long TAI should be used because most of LARS patients who were treated by TAI suffer from their symptoms refractory to treatment as shown in many studies.[66,67,70] Martellucci et al[70] in their study, they suspended the use of TAI after follow up period of 6 months and advised patients to use regular enemas instead. Unfortunately, 85% of the patients returned to a TAI due to rapid recurrence of LARS symptoms. It is important to note that TAI only provides a state of pseudocontinence by preventing fecal leaks between washouts.[72] The major key to success of TAI is professional training.[73]
Pelvic floor rehabilitation includes biofeedback, pelvic floor muscle training, electrostimulation, and rectal balloon training. Using of more than one technique has proven to significantly improve outcomes than individual techniques.[74] As it has been used for treatment of FI, so biofeedback may be effective for treatment of patients with LARS because the main symptom of LARS is FI. Biofeedback therapy increases the rectal sensation of distension and synchronous voluntary external anal sphincter contraction in response to rectal distension.[72] Although the available studies on the role of biofeedback and pelvic floor rehabilitation are heterogeneous and of low quality, it is still beneficial in the treatment of LARS. Kim et al[75] reported that biofeedback therapy improves the FI score, number of bowel movement, resting pressure, SP, and rectal capacity by anorectal manometry in patients who were subjected to SPS for rectal cancer. In contrast, anorectal evaluation by manometry was performed in two studies and did not show any change in the rectal pressures after pelvic floor rehabilitation.[74,75] Liang et al[76] in their study, evaluated the value of biofeedback in patients with LARS, and their results showed improvement in FI scales, number of bowel movements, and anorectal manometry. Moreover, Ridolfi et al[77] recommended the use of biofeedback therapy as one of the best treatment options in LARS.
If symptoms of LARS persist for more than 1 year, SNS should be considered.[59] SNS decreases the postprandial urgency, enhancing the ability to hold stool and so improving patients’ QoL and anorectal function by both pelvic afferent and central mechanisms.[78] With acceptable safety, the SNS significantly improves the FI episodes of LARS which is quite similar to its effect on all causes of FI.[72] In one study by Eftaiha et al[79] significant improvement in the Cleveland Clinic incontinence scores and LARS scores at a median follow up of 19.5 months after using SNS. Dramatic improvement in the Wexner and LARS scores was reported by D’Hondt et al[80] in their study, emphasizing that SNS is effective for LARS symptoms improvement.
Other newly developed techniques of neurostimulation including posterior tibial nerve stimulation (PTNS) and electro-acupuncture are still under investigation for treatment of FI symptoms of LARS.[59] Percutaneous tibial nerve stimulation is one of the treatment methods for FI improvement.[81] Through indirect low voltage stimulation of the tibial nerve at the ankle, PTNS can regulate sacral nerve function and is less invasive and cheaper than SNS.[72] In a pilot study by Altomare et al[81] including 21 patients, the number of FI episodes was decreased in nine patients after PTNS.
Patients who still not respond to the previously mentioned modalities may require surgery. Surgery includes either artificial sphincter-which is associated with higher complication rate or fecal diversion which is the last option especially when the patient still has major LARS symptoms after 2 years of conservative or minimally invasive treatment (SNS, PTNS).[59]
There is still debate about the time of improvement of bowel function after SPS. It has been reported that the symptoms of bowel dysfunction mostly occur during the first year after surgery and then become more stabilized as Perez et al reported.[82] In contrast, another study reported that 47.5% of patients still had LARS symptoms even after a follow-up period of 13.7 years. Moreover, one study reported major LARS in 46% of the patients with a mean follow-up of 14.6 years and this may be due to other associated risk factors.[83] Hence, the need for further prospective longitudinal studies for further assessment of this point is mandatory.
It is difficult to prevent the occurrence of LARS after SPS. However, every effort should be exerted to decrease its incidence. Preserving the hypogastric nerve during ligation of the inferior mesenteric artery and the pelvic autonomic plexus during TME is mandatory.[84] Creation of a neorectal reservoir can alleviate LARS symptoms.[21] Many techniques can be used including colonic J pouch, end-to-side anastomosis, transverse coloplasty with comparable functional results. Both the coloplasty and the end-to-side anastomosis are less bulky. However, the coloplasty has a higher risk of AL, hence J pouch and end-to-side anastomosis are preferred.[85] It is mandatory to discuss with the patients pre-operatively about the possible complications related to bowel function and its effect on the QoL. Addressing the risk factors before surgery and proper patient selection can mitigate the LARS symptoms. Additionally, Battersby et al administered a new tool: the pre-operative LARS score (POLARS) which is the first nomogram and online tool to predict bowel dysfunction severity before surgery. Colorectal surgeons, gastroenterologists, and nurse specialists may use POLARS to help and discuss with the patients to understand the risk of bowel dysfunction and to decide which patient will need additional post-operative support.[86]
With SPS, LARS is an inevitable consequence. Scoring systems can be used such as the LARS, MSKCC scores along with objective testing tools, to assess the patient’s functional status. Patients with minor LARS can be managed with medication and pelvic floor rehabilitation, but patients with major LARS should be treated with more aggressive multimodal treatment like pelvic floor rehabilitation and neuromodulation. If the severities of the symptoms persist for more than 2 years, permanent stoma formation should be carefully considered after patient’s consent.[87]
Pre-treatment patients’ counseling is an important step for those who have risk factors of LARS. Proper selection of preoperative chemoradiotherapy, meticulous surgical dissection in the anatomical planes, trying to create a neorectal reservoir during surgery, proper anal sphincter Kegel exercise, and appropriate diet control are essential to minimize the incidence or to alleviate LARS. Finally, a very crucial point is the good decision before choosing SPS especially for low rectal cancer based on the balance between the oncological outcome that must be the primary aim and the QoL. In the future, more prospectively collected data in a longitudinal standardized manner for better assessment of the functional outcomes after SPS is needed.
None.






























