
胃癌是严重危害我国人民健康的消化系统恶性肿瘤。近年来,免疫治疗在对胃癌等多个癌种的治疗中异军突起,成为继手术、放化疗和分子靶向治疗之后的另一种有力手段,极大延长了晚期胃癌患者的生存期且逐步应用于进展期胃癌。然而,不同患者对免疫治疗的响应具有较大异质性,并非所有患者都能从免疫治疗中获益。如何在治疗前对患者进行有效的筛选,避免免疫治疗无效的患者接受不必要治疗是胃癌免疫治疗亟待解决的重大难题之一。目前,已经有一些生物标志物用于对胃癌免疫治疗响应的预测,但临床效果不佳,开发可靠有效生物标志物用于筛选免疫应答响应的患者群体至关重要。本文就目前几种临床应用较广泛的胃癌免疫治疗疗效预测的生物标志物进行综述,探讨其目前的应用现状及面临的问题、挑战及发展趋势。未来开发多个生物标记物联合评估模式及预测模型的建立可能更准确地筛选胃癌免疫治疗优势人群,进一步推动未来胃癌精准个体化免疫治疗的实现。

版权归中华医学会所有。
未经授权,不得转载、摘编本刊文章,不得使用本刊的版式设计。
除非特别声明,本刊刊出的所有文章不代表中华医学会和本刊编委会的观点。
全球范围内,胃癌发病率逐年升高,已经成为发病率排名第五、死亡率排名第四的恶性肿瘤[1]。手术、化疗、放疗、靶向治疗等综合疗法在胃癌治疗方面取得了较大进展,但胃癌患者的5年生存率仍然很低。在我国约75%的胃癌患者初诊时已属晚期,失去手术机会[2]。局部进展期胃癌及转移性胃癌的治疗选择有限加之对传统化疗耐药致使此类患者预后不良,因此迫切需要新的治疗方法来改善这些患者的预后。慢性感染与恶性肿瘤之间的关联在胃癌中已得到广泛认可,基于此有学者提出靶向免疫系统或可改善对传统化疗耐药的肿瘤患者的预后[3, 4, 5]。免疫疗法旨在增强免疫系统自然防御能力以消除恶性细胞,是癌症治疗的巨大突破,是目前癌症治疗领域的“风向标”。以免疫检查点抑制剂为代表的新型免疫疗法在进入我国的5年中蓬勃发展,已成为多种晚期癌种的治疗基石,被称为肿瘤治疗的又一里程碑。多项研究证实免疫治疗相关药物可为肿瘤患者带来显著的长期生存获益及优势的治疗反应,实体瘤(如黑色素瘤、非小细胞肺癌和泌尿生殖系统肿瘤)和血液系统恶性肿瘤的治疗范式已经发生了根本性的转变[6, 7, 8]。
然而,不同患者对于免疫治疗的响应具有较大异质性,并非所有患者均能从免疫治疗中获益。晚期胃癌患者对于免疫治疗的响应率在帕博利珠单抗或纳武利尤单抗单药治疗的临床试验中表现出了较大的差异,客观缓解率为10%~26%[9, 10, 11]。如何在治疗前对患者进行有效筛选,避免免疫治疗无效的患者接受不必要治疗是胃癌免疫治疗领域亟待解决的重大难题之一。本文就目前应用较广泛的胃癌免疫治疗疗效预测的生物标志物进行综述,以期为临床诊疗提供参考。
1. 肿瘤突变负荷(TMB):癌症是一种遗传性疾病,体细胞突变事件的累积导致肿瘤的发生发展[12]。在某肿瘤不同部位及不同肿瘤类型之间,每种类型的突变频率存在差异[13]。肿瘤DNA中部分体细胞突变可以产生新抗原,从而被免疫系统识别和靶向清除。TMB定义为肿瘤基因组中体细胞突变数量,TMB高的肿瘤细胞将具有更高水平的新抗原,有助于免疫系统识别肿瘤并刺激抗肿瘤T细胞的增殖,因此目前认为TMB可能与免疫治疗应答率正相关[14]。Cristescu等[15]研究认为TMB≥175 mut/Mb与帕博利珠单抗单药治疗疗效的提高以及帕博利珠单抗与化疗联用在晚期实体瘤治疗中疗效的改善相关。他们认为TMB在多种癌种中均具有广泛的临床应用价值,并支持其在既往治疗过的晚期实体瘤患者中作为帕博利珠单抗单药治疗的预测生物标志物。已有多项研究证实了胃癌中TMB水平与患者总体生存率及临床获益相关[16, 17]。然而McGrail等[18]对超过10 000例患者肿瘤的数据进行分析后认为高TMB可能不适合作为所有实体瘤类型的免疫治疗的生物标志物,需要进一步的肿瘤类型特异性研究加以证实。
2. 微卫星不稳定(MSI)错配修复基因缺失(MMR):MSI是指由于在DNA复制时插入或缺失突变引起的微卫星序列长度改变,常由dMMR引起。MSI状态是目前公认的免疫治疗反应预测生物标志物,可分为高度MSI(MSI-H)、低度MSI(MSI-L)及微卫星稳定(MSS)。通常认为MSI-H患者体内具有相对更高的基因突变率及更多肿瘤新抗原的产生,Vanderwalde等[19]提出MSI-H的患者大部分呈现高TMB状态。研究表明[20]在胃或食管交界癌中,MSI-H发生率为5%~20%。已有多项临床试验证实MSI-H可以作为胃癌免疫治疗疗效预测的潜在生物标志物:KEYNOTE-059研究发现帕博丽珠单抗治疗MSI-H胃癌患者的客观缓解率高于非MSI-H胃癌患者(57.1%比9.0%)[21];KEYNOTE-061研究结果提示相比于紫杉醇,帕博丽珠单抗治疗MSI-H胃癌患者可有更多获益[22]。一项纳入1 556例胃癌患者的荟萃分析指出,MSI-H胃癌患者5年无病生存率为71.8%,5年总生存率为77.5%;而MSS/MSI-L患者的5年无病生存率为52.3%,5年总生存率为59.3%[23]。在可切除的原发性胃癌患者中,MSI-L/MSS胃癌患者可从化疗联合手术中获益,相反MSI-H的胃癌患者则没有明显获益[22]。以上结果提示在MSI-H胃癌患者中,传统化疗可能存在潜在危害,对此类患者开展免疫治疗或可改善其预后结局。
3. 特定基因突变:某些癌基因及抑癌基因的突变、缺失等不同形式与免疫治疗疗效密切相关。检测肿瘤患者中这些新型突变基因的存在有助于临床指导用药,进而进一步探索其临床应用前景。POLE及POLD1基因是参与DNA修复的重要基因,有研究表明,存在POLE或POLD1基因突变肠癌、子宫内膜癌及肺癌患者接受免疫治疗后肿瘤退缩显著,可作为免疫检查点抑制剂治疗中的独立预测因子[24, 25];ARID1A突变及人类白细胞抗原分型有望成为胃癌免疫治疗效果的潜在生物标志物[26]。核因子κB(NF-κB)相关代谢基因有望指导胃癌免疫治疗及预测预后[27]。干扰素(IFN)-γ信号通路及T细胞活化相关基因可能成为预测胃癌免疫疗效的潜在标志物[28]。目前胃癌免疫治疗领域的基因突变研究相对有限,未来仍需大量临床试验及转化研究探索其临床前景。
程序性细胞死亡受体1(PD-1)在多种细胞中广泛表达,PD-L1在抗原呈递细胞和多种肿瘤细胞膜上表达。与PD-1结合后,PD-L1可以持续激活免疫抑制剂信号通路,逃避免疫系统监测,促进肿瘤逃逸[2]。PD-L1通路因其在引发T细胞免疫检查点反应中的作用而受到广泛关注,导致肿瘤细胞对常规化疗具有高度耐受性。免疫检查点抑制剂已成为多种恶性肿瘤的一线治疗药物,迄今为止,PD-1/PD-L1检查点阻断疗法已成为包括黑色素瘤、非小细胞肺癌、肾细胞癌、结直肠癌、肝细胞癌等恶性肿瘤的标准疗法之一,多项临床试验正深入研究其治疗其他恶性疾病的可行性[29]。
目前在胃癌免疫治疗领域应用的抗PD-1/PD-L1药物主要有纳武利尤单抗、派姆单抗等。研究表明,PD-L1在12%~65% 的胃癌患者中的表达上调,且高表达PD-L1的胃癌患者预后较差[30, 31]。一项多中心Ⅲ期临床试验KEYNOTE-59发现,接受派姆单抗治疗的PD-L1阳性晚期胃癌患者的客观缓解率高于PD-L1阴性患者(68.8%比37.5%)[32];KEYNOTE-012试验招募了39例晚期胃癌患者,使用帕博利珠单抗治疗后,中位总生存时间为11.4个月[33]。此外,该研究还表明,PD-L1表达与总生存期相关,也可作为临床肿瘤复发监测指标。以上结果提示PD-L1高表达的患者可能会从抗PD-1/PD-L1治疗中获益更多。目前临床上主要通过免疫组织化学染色检测PD-L1的表达,联合阳性分数(CPS)用于准确评价PD-L1表达水平。有研究表明CPS评分较高的胃癌患者接受免疫治疗后的客观缓解率更高,在总体生存率中也表现出了明显优势[34]。KEYNOTE-061研究纳入395例CRS评分≥1的胃癌患者,发现CPS≥10分的患者在帕博丽珠单抗治疗中获益更大(中位生存时间为10.4个月)[22]。KEYNOTE-062研究也观察到了相似结果[9]。最近的1项研究综合3项Ⅲ期试验数据(CheckMate-649、KEYNOTE-062和KEYNOTE-59)探究低PD-L1 CPS亚组的预后情况;结果表明,PD-L1低表达患者不能从免疫治疗联合化疗中获益[35]。然而由于不同类型试剂盒对于PD-L1的评价效能及标准存在差异,PD-L1作为预测胃癌免疫疗效的指标仍需进一步深入探索。
近年来,由于TME在调节肿瘤进展和治疗方法的反应方面起着关键作用,针对TME的治疗策略已成为癌症免疫治疗的热点。有学者发现[36]抗原提呈细胞上PD-L1的表达,可以控制抑制性调节T细胞的分化和抑制其活性。然而,肿瘤细胞和其他TME成分,如浸润髓系细胞和树突状细胞,能够通过上调PD-1配体来诱导T细胞衰竭,产生免疫抑制TME促进肿瘤生长和侵袭[37]。免疫治疗的响应大多依赖于肿瘤细胞与TME免疫调节剂的相互作用。Cañellas-Socias等[38]发现结直肠癌中存在高复发能力的肿瘤细胞,其所在的转移性TME中T细胞的浸润显著增多,因此术前新辅助免疫治疗可能会显著降低术后的转移性复发风险。Zeng等[39]建立微环境评分TME score以量化肿瘤组织免疫激活状态,进而预测免疫治疗的疗效。他们进一步利用多中心的临床队列验证TME score的预测价值,结果显示不同TME特性可以预测胃癌患者对免疫治疗的疗效[40]。中山大学肿瘤防治中心近期发表研究称联合PD-L1表达与肿瘤免疫微环境分型的评分模型可准确预测晚期非小细胞肺癌免疫治疗疗效[41]。夏楠等[42]研究证实CXCL13+CD8+T细胞浸润可作为胃癌患者独立的预后和预测指标。本课题组前期研究结果显示,胃癌TME中存在广泛的细胞重塑,且胃癌TME存在的特殊的巨噬细胞Mφ_APOE参与了胃癌免疫抑制微环境的塑造[43];此外,本课题组发现Tc17细胞同样影响胃癌TME。以上提示对胃癌TME的探索可能为精准免疫治疗寻找有价值的标志物。
1. 外周血生物标志物:外周血采集简单可行,相比复杂有创的组织活检,是近年来常用的肿瘤随诊评估手段。嗜酸性粒细胞的比例在肿瘤患者外周血中显著增高[44],Carretero等[45]报道嗜酸性粒细胞可能通过促进TME中CD8+浸润进而诱导肿瘤退缩,表明嗜酸性粒细胞可能参与促进免疫系统的抗肿瘤反应。我国学者发现外周血嗜酸性粒细胞比例升高与晚期实体瘤患者免疫治疗后生存率呈正相关,提示其可能是免疫治疗疗效的阳性标志物[46]。Namikawa等[47]研究报道接受纳武利尤单抗治疗4周的胃癌患者,完全缓解或部分缓解组中性粒细胞与淋巴细胞比值显著低于稳定期或进展性疾病组(2.2比2.9,P=0.044)。同时,他们发现纳入血清白蛋白及C反应蛋白的格拉斯哥预后评分可能是纳武利尤单抗晚期胃癌疗效的有用预测标志物[47]。
2. 液体活检生物标志物:据报道,循环肿瘤DNA(ctDNA)在识别基因突变、预测预后、识别靶向治疗的耐药性以及监测肿瘤的复发方面意义重大[48]。更重要的是,ctDNA可以动态监测肿瘤特异性遗传特征,无需重复进行侵入性肿瘤活检。Jin等[49]纳入46例免疫治疗联合化疗的晚期胃癌患者,结果发现ctDNA检出率与组织高度匹配且ctDNA丰度下降是预测免疫治疗疗效的有效预测因子。另有一项临床研究证实ctDNA丰度下降的胃癌患者预后良好。以上研究揭示了ctDNA与胃癌免疫治疗疗效的相关性,为临床应用ctDNA是动态监测肿瘤复发及疗效预测提供参考[50]。
近年来,影像学生物标志物及影像组学成为肿瘤患者非侵入性评估的有前景工具,可以全面分析TME,疾病的空间异质性及其时间变化[51]。研究[52, 53]证实CT影像组学特征可以预测包括胃癌在内的多种晚期实体瘤对免疫治疗的治疗响应。CT/MRI显像仅能提供肿瘤的解剖学信息,无法提供肿瘤免疫应答相关分子水平信息[54]。核医学分子影像具有在体、动态、无创、定量可视化免疫相关标志物的优势,为肿瘤免疫效果监测提供了新的手段。Bensch等[55]发现89Zr标记的抗PD-L1抗体肿瘤摄取率与患者预后呈正相关。考虑到肿瘤内PD-L1表达异质性,他们进而证实89Zr标记的抗CD8单臂抗体与免疫疗效密切相关[56]。我国学者开发了新型小分子探针68Ga-grazytracer 实现了CD8+T细胞的在体稳定显像,在多种肿瘤模型中实现无创有效地预测肿瘤对免疫检查点抑制剂和过继性T细胞转移疗法的反应[57]。近年来,多项研究利用放射性核素手段可视化体内激活型T细胞,进而监测肿瘤疫苗、免疫检查点阻断的治疗效果[58, 59]。Gibson等[60]利用放射性标记的抗IFN-γ抗体显像作为肿瘤疫苗的疗效预测标志物。
1. EB病毒:EB病毒相关胃癌(EBVaGC)是胃癌的一种独特分子亚型,约占全球胃癌的5%~10%,预后良好[61, 62]。晚期EBVaGC患者对抗PD-1单抗的反应率为25%~100%[63]。据报道,EB病毒感染会增加肿瘤浸润淋巴细胞和免疫检查点分子的表达,从而引起对免疫检查点抑制剂的反应,因此目前临床上针对EBVaGC患者首选免疫治疗[64]。2018年韩国学者对6例EB病毒阳性的转移性胃癌患者采用帕博利珠单抗治疗,客观缓解率高达100%[50]。EBVaGC瘤内存在ARID1A、PIK3CA和AR的高突变率[62],以上突变与实体瘤抗肿瘤免疫应答及对免疫检查点抑制剂的敏感性的提高有关;而EB病毒阴性患者具有更高的TP53突变率,已知TP53突变与胃癌患者的免疫疗效较差有关,以上结果提示EB病毒阳性患者比EB病毒阴性患者从免疫治疗获益的可能性更大。
2. 免疫相关不良事件(irAEs):免疫检查点抑制剂导致免疫细胞产生持久抗肿瘤反应的同时,还会引发免疫系统失衡从而导致与经典化疗毒性不同的irAEs[65]。来自日本的一项研究发现接受纳武利尤单抗治疗后出现irAEs的胃癌患者有更大的临床获益[66]。尽管irAEs对胃癌免疫检查点抑制剂疗效的确切影响和潜在机制仍有待阐明,但EB病毒感染作为一种胃癌免疫疗效预测的新兴生物标志物有望为胃癌患者个性化管理提供更多依据。
表1总结了目前临床应用较广泛的免疫治疗疗效预测的生物标志物的主要特点。除此之外,尚有大量标志物用于胃癌领域,例如性别[67]、体质指数[68]、肠道微生物组生物标志物[69]、幽门螺杆菌[70],肿瘤浸润淋巴细胞等[10,71]。
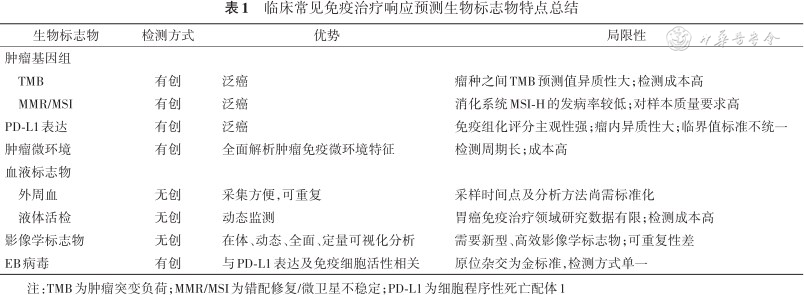
临床常见免疫治疗响应预测生物标志物特点总结
临床常见免疫治疗响应预测生物标志物特点总结
| 生物标志物 | 检测方式 | 优势 | 局限性 |
|---|---|---|---|
| 肿瘤基因组 | |||
| TMB | 有创 | 泛癌 | 瘤种之间TMB预测值异质性大;检测成本高 |
| MMR/MSI | 有创 | 泛癌 | 消化系统MSI-H的发病率较低;对样本质量要求高 |
| PD-L1表达 | 有创 | 泛癌 | 免疫组化评分主观性强;瘤内异质性大;临界值标准不统一 |
| 肿瘤微环境 | 有创 | 全面解析肿瘤免疫微环境特征 | 检测周期长;成本高 |
| 血液标志物 | |||
| 外周血 | 无创 | 采集方便,可重复 | 采样时间点及分析方法尚需标准化 |
| 液体活检 | 无创 | 动态监测 | 胃癌免疫治疗领域研究数据有限;检测成本高 |
| 影像学标志物 | 无创 | 在体、动态、全面、定量可视化分析 | 需要新型、高效影像学标志物;可重复性差 |
| EB病毒 | 有创 | 与PD-L1表达及免疫细胞活性相关 | 原位杂交为金标准,检测方式单一 |
注:TMB为肿瘤突变负荷;MMR/MSI为错配修复/微卫星不稳定;PD-L1为细胞程序性死亡配体1
免疫治疗的兴起开启了胃癌治疗的新模式,然而免疫疗法响应率低及缺乏预测疗效的生物标志物是目前面临的重大挑战。目前,已经有一些生物标志物用于对胃癌免疫治疗响应的预测。然而在个体水平上使用这些生物标志物的多个临床试验显示出了不一致的效能结果,有些甚至产生了矛盾的结论[72]。迄今为止,我们仍缺乏在胃癌中用于指导免疫治疗响应预测及用药选择的可靠普适的生物标志物。胃癌免疫治疗领域尚需更多研究深入开发可靠有效分子标志物,进一步推动未来胃癌精准个体化免疫治疗的实现。
姜玉娟, 田艳涛. 胃癌免疫治疗疗效预测相关标志物的研究进展[J]. 中华医学杂志, 2024, 104(16): 1431-1436. DOI: 10.3760/cma.j.cn112137-20231016-00769.
所有作者声明不存在利益冲突



























