
肝门部胆管癌严重危害国人健康,在我国发病率和病死率均呈上升趋势。根治性手术切除是肝门部胆管癌唯一可能治愈的有效手段,但由于患者确诊时多为晚期,且肝门部解剖结构复杂、高位胆管肿瘤无法精准定位,根治性手术切除率低,致使我国肝门部胆管癌患者的远期生存率远低于国际领先水平。随着高精度影像检查方法和内镜下活组织检查的广泛开展,现已能在术前精确评估肝门部胆管癌的侵袭范围,然而术中高位胆管离断点的确定仍然只能依赖术者的经验,根据肝门部胆管与血管的解剖位置关系以及触诊的胆管软硬度来判断。临床迫切需要术中影像学辅助设备对肝门部胆管癌的侵袭范围进行实时成像,并精准引导胆管离断,从而提高根治性切除率,减少术后并发症发生率,进而提高肝门部胆管癌的远期生存率。笔者总结肝门部胆管癌的术前规划方法及术中导航技术的现状,并对胆管腔内超声检查在术中实时引导胆管离断的国际首次应用实例作出分析,旨在为术中导航技术的发展提出展望。

版权归中华医学会所有。
未经授权,不得转载、摘编本刊文章,不得使用本刊的版式设计。
除非特别声明,本刊刊出的所有文章不代表中华医学会和本刊编委会的观点。
肝门部胆管癌是一种源自胆管上皮的高度异质性恶性肿瘤[1]。由于其早期缺乏特征性临床表现,大多数患者在确诊时已为不可切除的晚期,预后极差,病死率逐年上升。根治性手术切除是唯一可延长患者长期生存时间的方法。术前精确评估肝门部胆管癌的侵袭范围,术中确定高位胆管离断点位置,对影响肿瘤复发与患者长期生存有重要作用。依赖于影像学引导的手术增强了术中肿瘤边界和无瘤边界的精确定位。近年来,内窥镜技术和术中超声检查技术的发展为肝门部胆管癌手术导航提供了新契机。笔者总结肝门部胆管癌的术前规划方法及术中导航技术的现状,并对胆管腔内超声检查在术中实时引导胆管离断的国际首次应用实例作出分析,旨在为术中导航技术的发展提出展望。
肝门部胆管癌的最佳治疗方案是根治性手术切除(R0切除),但R0切除率仍有待提高[2, 3]。术前评估肿瘤的轴向浸润和侧方浸润范围对于制订手术计划至关重要,胆道狭窄的延伸情况可提供肿瘤沿胆管树轴向浸润的信息,从而确定相应的分期;对肿瘤侧方浸润范围的评估主要根据肝动脉、门静脉、肝实质等受累情况[4]。影像学方法可为术前手术规划提供重要信息。
肝门部胆管癌侧方浸润范围、软组织浸润及血管包绕情况依赖于多排螺旋CT检查,其对于可切除性评估的准确率为74.5%~94.4%[5]。用三维重建的方法处理多排螺旋CT扫描检查所得数据,可用于直接浸润及肝转移、淋巴结转移、周围神经侵犯的诊断[6]。伴有梗阻性黄疸的肝门部胆管癌,首先进行多排螺旋CT检查,根据肿瘤位置行右侧肝切除或左侧肝切除术,再通过经皮经肝胆管引流术或者内镜鼻胆管引流术对拟保留侧的胆管进行引流,并利用引流管进行直接胆管造影检查。经皮经肝胆管引流术和内镜鼻胆管引流术可降低胆红素,改善剩余肝脏的储备功能,纠正严重营养不良和凝血异常[7]。胆道梗阻导致高胆红素血症的患者,术前应评估肝储备功能及剩余肝体积能否耐受联合大范围肝切除。功能性剩余肝体积可通过CT检查及三维重建软件进行测量,应保留≥40%的正常肝实质,通常以引流后TBil降低速度进行评估。
肝门部胆管由于解剖关系复杂常需要个性化地选择多种成像方法,通常通过MRCP或直接胆管造影检查进行评估。先参照MRCP检查结果显示的胆管树形态进行总体判断,根据CT和MRI层析成像检查结果显示的胆管壁增厚和增强征象进一步分析和判断肿瘤与正常胆管组织之间的边界。MRI联合MRCP检查进行术前分期评估的准确性与多排螺旋CT联合直接胆管造影检查的准确性相似,但前者的优势在于可以避免侵入性操作和造影剂的使用[8]。经皮经肝胆管造影术和ERCP可作为MRCP的替代方法,用于显示胆管癌的浸润程度。
由于肝门部胆管癌存在黏膜下浸润及黏膜层扩展,故依据影像学检查精确判断胆管轴向浸润范围常存在困难。内镜下胆管上皮多点采样活组织病理学检查并结合胆道三维重建影像定位,有助于提高对肿瘤轴向扩展程度判断的准确性。
经皮经肝胆道镜检查可用于胆管狭窄的诊断与治疗、准确诊断胆管癌。经皮经肝胆道镜检查可详细观察胆管内腔和直视下行黏膜活组织检查,对诊断胆管癌黏膜内进展范围、确认胆管切断位置有重要作用[12]。对于狭窄的下游侧在胆道造影检查时不显影,经皮经肝胆道镜检查时可用胆道镜抵在狭窄中心,经导管扩张,行下游侧胆管黏膜活组织检查。
EUS可评估胆囊及胆管病灶的局部侵犯情况、肝门区淋巴结和血管侵犯情况[13]。其与细针吸取取样相结合,适用于非侵入性技术无法确诊的患者,总体特异度为92%~100%[14]。EUS联合细针吸取检查可明确肿瘤位置、判断管壁的侵犯深度,并对疑似淋巴结进行活组织检查。Malikowski等[15]证实EUS联合细针吸取检查可有效识别不同亚型胆道肿瘤患者的恶性淋巴结。由于抽吸针穿过十二指肠球部和腹腔取样会增加肿瘤扩散风险,因此,EUS仅用于无法进行根治性手术的患者,指导合适的一线治疗方案。
经口胆道镜可用于胆管癌轴向进展的诊断,多联合胆管腔内超声检查(intraductal ultrasonography,IDUS)行经乳头胆管活组织检查,通常在内镜下十二指肠乳头括约肌切开术或球囊扩张处理乳头部后将胆道镜导入胆管,需通过母镜(ERCP十二指肠镜)将子镜导入胆管,而经乳头插入胆道镜并越过狭窄部位的操作较为困难,目前临床不常用,但随着技术改良,管径更细的经口胆道镜应用将会更加广泛[16]。Bukhari等[17]曾报道联合使用经口和经皮经肝胆道镜直接引导下穿刺,可以精细操作穿刺针,降低出血和穿孔的风险。经口胆道镜的缺点在于检查需切开乳头进行,并且对于肿瘤上游侧进展程度诊断不充分。经口胆道镜包括直接胆道镜和间接胆道镜技术,目前临床上已出现多个经口胆道镜直视系统,其中常见的是直接经口胆道内镜、胆道子母镜系统、eyeMax洞察胆胰成像系统、SpyGlass数字单人操作胆道镜系统等[18]。Pereira等[19]报道使用SpyGlass DS对胆道狭窄患者进行评估并在直视下SpyBite活组织检查,诊断准确率为95.1%,灵敏度为100%、特异度为89.5%,该设备应用于肝门部胆管癌的诊断具有一定价值。
随着内窥镜技术的不断创新,SpyGlass DS数字单人操作胆道镜系统(Spy Glass DSOC)的问世,为肝门部胆管癌的术前肿瘤浸润范围边界划定提供了胆道成像、直视下活组织检查的技术选择,有利于术前肿瘤分型与手术方案的制订。近年来,内镜下活组织检查技术逐步提高,可在术前准确提供肿瘤病理学信息[20]。值得注意的是,Kurihara等[21]的1例病例报道SpyGlass成像质量低于视频胆管造影;在胆管内,由于SpyGlass的聚焦距离短,光量和分辨率有限,许多碎片和浓缩胆汁的存在影响了良好的观察效果。Chiang等[22]报道SpyGlass胆道镜可用于精确定位肝门部胆管癌,在手术前为外科医师提供更多信息。SpyGlass胆道镜检查实现了胆总管和胆道的可视化,可提供高分辨率的胆管图像,有望在肿瘤精确定位方面发挥作用[22]。然而,由于肿瘤阻塞胆管,SpyGlass胆道镜检查难以在手术中进行,除非术前先扩张狭窄部位。因此,该技术还需进一步改进,但SpyGlass系统具有评估胆管肿瘤浸润范围的潜力。
腹部超声检查用于确认可疑的胆管梗阻,并确定狭窄的位置和程度。超声检查在局部进展程度的诊断上,可显示肝内胆管根部以了解肿瘤轴向进展程度,显示肝门部肿瘤并了解对动脉、门静脉和肝实质侧方浸润程度[23]。术中超声检查利用高频探头和直接接触扫描的两大优势,可以近距离观察肝内外胆管系统的形态以及与周围组织的关系,也可在术中判断有无肝转移、脉管侵犯以及淋巴结转移,进而确定肿瘤的可切除性,以及对肝门部胆管癌进行术中分期[24, 25]。术中超声检查可根据肝内胆管扩张与狭窄部位初步判断胆管离断点并以钛夹或钳夹作为标记[26]。
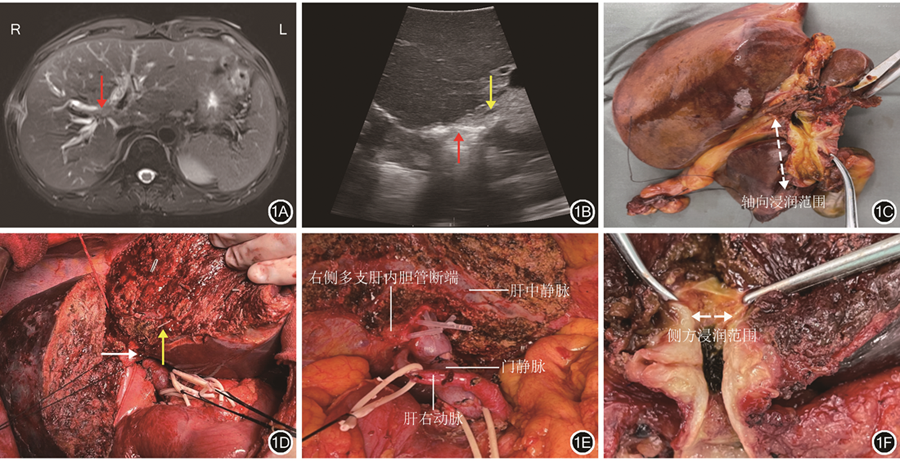
由于劈开肝脏后肝门部胆管已完全显露,气体干扰和难于耦合使得术中探头,无论是经过肝脏还是直接放置于胆管表面,都不能清晰显示胆管壁各层结构,因此,开腹术中超声检查只能进行粗略导航。示例1:患者男,63岁,发现皮肤、巩膜黄染2周入院,术前MRI检查结果提示肝门部胆管占位并肝右叶占位。有糖尿病史。行肝动脉受累肝门部胆管癌扩大根治术,术中切除右半肝及肝外胆道,行门静脉及肝动脉重建。63岁男性患者术前影像学检查结果及开腹术中超声检查结果见图1。

术前通过SpyGlass DS胆道镜直视化技术对肿瘤的侵袭边界划定及活组织检查后,通常会进行IDUS以确定肿瘤的侵犯范围,IDUS检查以微型超声探头经十二指肠侧插入胆道分支进行胆总管扫查,可清晰显示胆管和胰管腔内、管壁及其三维图像,并诊断黏膜和黏膜下的延伸范围[27, 28, 29]。IDUS检查在判断胆道狭窄的良、恶性和诊断胆管癌方面具有较高的准确性。狭窄段的IDUS图像具有沿导管周围组织浸润性病变、胆管壁不规则增厚和外回声壁层中断的特征[30]。根据IDUS检查结果,并结合胆管活组织检查,可确定浅表肿瘤扩展程度。
IDUS检查术前可帮助术者判断肿瘤的扩展程度,从而制订手术方案,但尚未有其在术中实时引导的病例报道。笔者团队已在国际上首次将IDUS检查应用于肝门部胆管癌术中引导胆管离断点,并已成功完成6例。示例1:患者女,82岁,发现胆管占位1周入院,有高血压、糖尿病史。术前MRI检查提示肝左叶近肝门部肿瘤。行肝动脉受累肝门部胆管癌扩大根治术,术中切除左三肝,肝右动脉受累切除重建。患者术前影像检查结果及术中IDUS检查图像显示见图2。
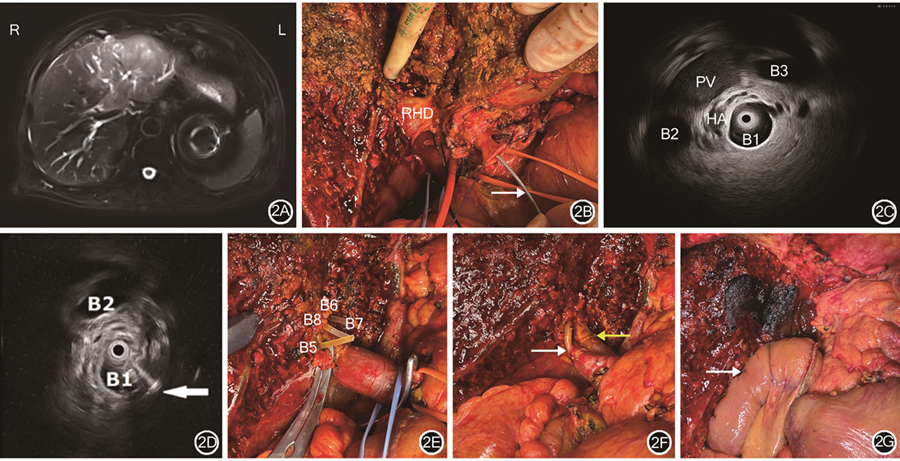

注:IDUS为胆管腔内超声;RHD为右肝管;PV为门静脉;HA为肝动脉;B1为IDUS探头所在右肝段胆管;B2、B3为正常右肝段胆管;B5为肝Ⅴ段胆管;B6、B7为肝Ⅵ、Ⅶ段胆管;B8为肝Ⅷ段胆管
随着影像学技术的飞速发展,术前手术规划已经逐渐完善,现阶段术中导航技术的革新已经逐渐成为肝门部胆管癌手术的焦点。术中超声检查技术对于肝门部胆管癌肝内转移病灶的鉴别与胆管解剖结构的分辨有巨大价值,而笔者在术中进行胆管腔内超声的首次应用,为我们提示了该技术新的发展潜力,若进一步提升IDUS检查应用的场景与精准度,有望在肝门部胆管癌精准手术导航中取得突破性进展。
王宏光, 罗漫. 肝门部胆管癌的术前评估和术中导航研究进展[J]. 中华消化外科杂志, 2024, 23(7): 906-911. DOI: 10.3760/cma.j.cn115610-20240612-00291.
所有作者均声明不存在利益冲突



























