
局灶性白质损伤(punctate white matter lesions,PWML)是婴儿期最为常见的白质损伤性疾病(发生率超过20%),可引起不良神经发育结局,严重威胁患儿的身心健康发展。该损伤具有多变性及扩展性,可导致广泛脑结构及功能变化。笔者主要阐述了PWML病灶特征及其预后评估方面的研究进展,指出现有损伤评估中存在的问题并进行展望。

本刊刊出的所有论文不代表本刊编委会的观点,除非特别声明
孕晚期及生后早期是大脑快速发育的重要时期,但此时脑发育不成熟,极易受到宫内外各类致病因素扰动而出现脑损伤,其中以局灶性白质损伤(punctate white matter lesions,PWML)最为常见,发生率超过20%[1,2]。该损伤在常规MRI的表现为:分布于半卵圆中心及侧脑室旁白质内的点状、线状或簇状T1WI高信号,T2WI等或低信号[3,4]。PWML具有散在和多变的特点,可导致脑瘫、弱视及认知障碍等不良神经发育结局[5,6,7],严重影响儿童的身心健康发展。
在PWML被认为是一种特殊类型的损伤之前,经历了一系列概念的发展演变。20世纪90年代初,随着MRI在临床的应用,首次在新生儿头颅MRI图像中观察到这种分布于脑室周围、额叶和枕叶白质的T2WI低信号损伤[8]。2001至2002年,Cornette等[9]及Childs等[10]先后对这种局灶性损伤进行了定义和分级。随后,有学者将该损伤归为缺氧缺血性脑病的一种类型,并认为其可能与胎盘炎症反应程度有关[11,12,13,14]。随着MRI和神经病理学的发展,国内外学者根据其影像学和神经病理学的不同特征,先后将该损伤命名为脑室周围白质软化(periventricular leukomalacia,PVL)/非囊性PVL[15,16]、白质损伤[17]以及PWML[18,19]等。2017年,Volpe[20]将MRI表现与病理相结合,对该领域存在的多种命名方式进行了述评和解析,并提出用"白质损伤"来命名,其中PWML属于中度白质损伤。
目前,PWML病因尚不明确。临床多中心研究指出,较大的出生体重、Ⅲ-Ⅳ级脑室内出血及严重的并发症是发生PWML的独立危险因素[21]。也有文献报道PWML与先天性心脏病[22]、缺乏或不完全产前类固醇使用[23]、宫内及生后感染[24,25,26]、围产期窒息[25,27]及阴道分娩[28]等因素有关。由此可见,PWML可能为多因素共同作用的结果,其中以产时和产后因素为主。此外,有学者还发现剖宫产、晚期早产儿及正常出生体重是PWML的保护性因素[23,25]。
尸检研究表明,PWML在镜下表现为白质区域小坏死灶,伴病灶周围轴突肿胀、血管充血以及反应性胶质细胞增生[29,30,31]。随着对损伤病理生理的进一步研究,Miller等[32]指出,PWML主要是缺血缺氧或炎症作用下导致的少突胶质前体细胞受损。尽管损伤周围的少突胶质细胞可以代偿性增殖,但受增殖星形细胞产生的细胞因子影响,再生的少突胶质细胞发育成熟受阻,不能正常髓鞘化,最终引起白质发育延迟。除此之外,PWML还可阻碍神经元迁移及发育成熟,但不伴有轴突和神经元坏死。
为了明确能够准确评估PWML的最优序列,Liauw等[33]的研究表明,T1WI对PWML的检出明显优于相同层厚(4~ 5 mm)的T2WI、液体衰减翻转恢复序列、扩散加权成像以及T1WI增强序列,且具有较好的一致性。与ADC图(层厚4 mm,143个病灶)和磁敏感加权成像的幅度图(层厚2 mm,152个病灶)相比,3D-T1WI (422个病灶)对PWML的检出仍具有明显优势[34]。在不同层厚T1WI中,1 mm的3D-T1WI对PWML的检出(386个病灶)显著高于层厚3 mm的T1WI序列(218个病灶)[16]。尽管相位敏感反转恢复序列可以提高脑组织对比度和图像信噪比,其对PWML的检出效能(24个病灶)仍低于3D-T1WI序列(67个病灶)[35]。因此,高分辨率的3D-T1WI (层厚1 mm)为目前检测PWML的最优序列。
纵向随访研究表明,PWML在常规MRI上主要表现为随时间而逐渐消退的趋势,部分损伤类型可发生相互演变。早产儿矫正到足月时,PWML病灶数目减少、T1WI高信号减低[36],甚至在常规MRI上"消失"(22%~39%)[1,15]。当患儿有多于2个部位的重度感染或机械通气时间延长时,可显著增加PWML进展的风险[37]。线状和簇状PWML(中-重度)在后期则易发展为PVL[5]。由此可见,PWML病灶的动态变化不仅与损伤程度相关,而且与患儿临床病史及治疗方案关系密切。由此,生后及早行高分辨率MRI检查对PWML损伤程度的准确评估具有十分重要的临床意义。
基于病灶的量化分析表明,PWML区域ADC值在生后1周及2~5周内均显著低于病灶周围及对照组相同解剖区域,认为可能与损伤后细胞内水肿有关[38]。MRS研究表明,PWML区域谷氨酰胺较对照组显著升高,而N-乙酰天门冬氨酸(N-acetylaspartate,NAA)降低,推测与细胞外兴奋性毒性的谷氨酸水平继发性升高有关[39]。重T2*加权的三维梯度回波序列分析结果显示,病灶区R2*值高于对照组,可能与脑组织缺血缺氧后血管内脱氧血红蛋白含量升高、细胞肿胀、胶质细胞增生及再灌注后血浆蛋白渗出等一系列病理改变有关[40]。
除损伤病灶区域的变化外,PWML还可导致远隔部位白质微结构的广泛异常。基于DTI的分析显示,PWML可致内囊后肢、视辐射、大脑脚、小脑上脚及脑桥交叉纤维区域的髓鞘化延迟[31,41]。纤维束追踪技术进一步表明,PWML可引起投射纤维、联络纤维及联合纤维微结构属性的改变,且损伤类型与其距病灶的远近程度有关,反映了轴突与少突胶质细胞营养互助关系遭到破坏而导致的髓鞘化进程受阻及神经元胞体受损[2,19]。
PWML还可伴有灰质结构异常。基于常规MRI的视觉评估发现,PWML患儿皮层折叠程度减低,可能与白质损伤影响皮层神经元发育有关[42]。MRS在早期即可检测到PWML导致的深部灰质核团的神经元损伤,具体表现为丘脑NAA/Cho及NAA/Cr降低,豆状核NAA/Cho降低[43]。随着损伤进展,可出现丘脑体积减少,且体积减少的程度与PWML体积呈显著负相关[1,2]。丘脑-皮层连接发育于孕中晚期,与发生PWML的时间窗较为一致,因此损伤导致的丘脑-皮层连接的异常,使得丘脑内神经元成熟受阻而造成体积减少[44]。
功能MRI序列对扫描过程中的头部运动敏感,而新生儿耐受性差,难以配合者还需采用镇静药物,因此针对该人群的功能MRI研究较少。目前仅一项静息态功能MRI研究指出,PWML早产儿丘脑-突显网络的连接增加且以病灶较少的左侧明显,而丘脑-感觉运动网络连接无明显变化,增加的丘脑-突显网络连接可能由皮层功能代偿所致[45]。
脑血流及脑氧代谢水平是反映大脑生理功能的重要指标。基于相位对比和T2弛豫自旋标记技术的研究表明,PWML患儿氧摄取分数较对照组显著降低而静脉氧饱和度升高,认为其与缺氧(60.87%合并窒息、呼吸困难)导致神经元退变、坏死有关。同时,红细胞容积在PWML组显著增高,可能与窒息或呼吸困难导致细胞缺氧,进一步降低三磷酸腺苷,降低pH值和血流速度,使血液黏度增加有关[46]。
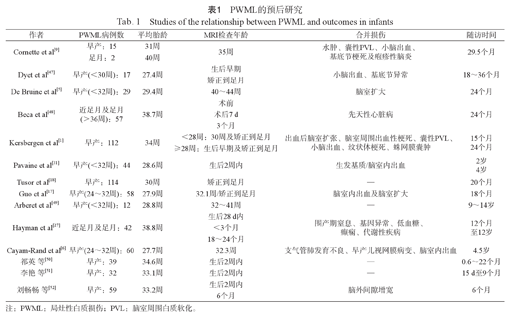
PWML发生率高,病灶分布散在且位置多变,已有多项研究对其预后进行评估(表1)。
PWML的预后研究
Studies of the relationship between PWML and outcomes in infants
PWML的预后研究
Studies of the relationship between PWML and outcomes in infants
| 作者 | PWML病例数 | 平均胎龄 | MRI检查年龄 | 合并损伤 | 随访时间 |
|---|---|---|---|---|---|
| Cornette et al[9] | 早产:15足月:2 | 31周40周 | 35周 | 水肿、囊性PVL、小脑出血、基底节梗死及疱疹性脑炎 | 29.5个月 |
| Dyet et al[47] | 早产(<30周):17 | 27.4周 | 生后早期矫正到足月 | 小脑出血、基底节异常 | 18~36个月 |
| De Bruine et al[5] | 早产(<32周):29 | 29.4周 | 40~44周 | 脑室扩大 | 24个月 |
| Beca et al[48] | 近足月及足月(>36周):57 | 38.7周 | 术前术后7 d3个月 | 先天性心脏病 | 24个月 |
| Kersbergen et al[1] | 早产:112 | 34周 | <28周:30周及矫正到足月≥28周:生后早期及矫正到足月 | 出血后脑室扩张、脑室周围出血性梗死、囊性PVL、小脑出血、纹状体梗死、蛛网膜囊肿 | 15个月24个月 |
| Pavaine et al[31] | 早产(<32周):44 | 28.6周 | 生后2周内 | 生发基质/脑室内出血 | 2岁4岁 |
| Tusor et al[18] | 早产:114 | 30周 | 矫正到足月 | — | 20个月 |
| Guo et al[17] | 早产(24~32周):58 | 27.9周 | 32.1周/矫正到足月 | 脑室内出血及脑室扩大 | 18个月 |
| Arberet et al[49] | 早产(<32周):12 | 28.8周 | 32~41周 | — | 9~14岁 |
| Hayman et al[27] | 近足月及足月:42 | 38.8周 | 生后28 d内<3个月18~24个月 | 围产期窒息、基因异常、低血糖、癫痫、代谢性疾病 | 12个月至12岁 |
| Cayam-Rand et al[6] | 早产(24~32周):60 | 27.7周 | 32.3周 | 支气管肺发育不良、早产儿视网膜病变、脑室内出血 | 4.5岁 |
| 祁英等[50] | 早产:39 | 34.6周 | 生后2周内 | — | 0.6~22个月 |
| 李艳等[51] | 早产:32 | 33.1周 | 生后2周内 | — | 15 d至9个月 |
| 刘畅畅等[52] | 早产:59 | 33.2周 | 生后2周内6个月 | 脑外间隙增宽 | 6个月 |
| 作者 | 评估量表 | 研究结果 |
|---|---|---|
| Cornette et al[9] | 粗大运动、社交、语言及精细运动适应性问卷 | 预后良好,轻度语言发育迟缓1例 |
| Dyet et al[47] | Grifths精神发育量表 | 预后良好 |
| De Bruine et al[5] | 粗大运动功能分级、贝利婴幼儿发展量表-Ⅲ、儿童行为量表 | 病灶数目≥6的患儿低的贝利评分、重度发育迟缓、脑瘫及更多的行为异常发生率高 |
| Beca et al[48] | 贝利婴幼儿发展量表-Ⅲ | 不良预后与脑发育不成熟有关,与PWML无关 |
| Kersbergen et al[1] | Grifths精神发育量表/贝利婴幼儿发展量表-Ⅲ | PWML类型及数目与预后无关 |
| Pavaine et al[31] | 贝利婴幼儿发展量表-Ⅲ贝瑞视觉运动整合量表 | 预后良好 |
| Tusor et al[18] | 粗大运动功能分级、贝利婴幼儿发展量表-Ⅲ | 中重度运动功能障碍,PWML体积越大运动功能评分越低 |
| Guo et al[17] | 贝利婴幼儿发展量表-Ⅲ | 额顶颞叶PWML体积越大运动预后越差,PWML位于额叶智力预后差 |
| Arberet et al[49] | 生活质量问卷 | PWML患儿运动障碍风险增加 |
| Hayman et al[27] | Grifths精神发育量表/贝利婴幼儿发展量表-Ⅲ韦氏学龄前/儿童智力量表 | 合并基因异常的PWML患儿预后差 |
| Cayam-Rand et al[6] | 韦克斯勒学前量表/儿童运动评估(第2版)/脑瘫神经系统评估 | 早期损伤位置可预测学龄前期发育结局(损伤位于前部预后差);加入矫正到足月时的临床指标可增强预测效能 |
| 祁英等[50] | 贝利婴幼儿发展量表-Ⅱ | 线状及混合型PWML易出现运动发育迟缓、认知障碍等 |
| 李艳等[51] | Gesell发育量表 | 点状PWML预后较好,线状、混合型及弥漫性预后较差 |
| 刘畅畅等[52] | Gesell发育量表 | 弥漫性、线状及混合性PWML预后较点簇状差 |
注:PWML:局灶性白质损伤;PVL:脑室周围白质软化。
PWML的预后研究主要集中于早产儿,且多在生后2周内和(或)校正到足月时行MRI扫描对损伤进行评估。由于纳入研究对象、MRI扫描时间、合并损伤以及预后评估时间的差异,对PWML发育结局判断并不一致。总体而言,其观点随着对PWML的深入认识而不断更新,主要分为以下3个阶段:(1) PWML预后良好,不会导致患儿发生明显后遗症。一方面,受早期研究小样本量(17例)的限制,其研究结果的可信度还有待考量[9,47]。另一方面,临床混杂因素以及合并损伤的严重程度也可能对发育结局的评估产生影响[1,48]。尽管如此,已有部分研究提示,簇状PWML可能会增加发生脑瘫的风险[1]。(2) PWML可致运动功能障碍,且病灶数目和体积与运动结局相关。当病灶数目≥6时,运动异常发生率增加[5];当PWML累及皮质脊髓束且病灶数目>20时,不良运动发育结局的风险明显增高(敏感性57%,特异性94%)[18]。与病灶数目相比,PWML体积是反映损伤程度更为客观的指标,尤其是针对病灶数目一致性较差的簇状病灶。因此,病灶体积为判断预后更为可靠的评估指标[17,18]。(3)PWML还可导致认知发育障碍,且病灶是否累及额叶是判断预后的重要指标。位于大脑前部的损伤病灶可有效判断学龄前期的不良认知(准确度90%)及运动(准确度85%)发育结局[6,17]。但该预测模型是基于极早产儿的重度PWML构建的,故其对不同PWML人群的适用性和准确性还有待进一步评估。
总之,PWML可导致不良运动及认知发育结局。其中运动发育结局与病灶体积相关,病灶体积越大运动预后越差,尤其是损伤累及双侧皮质脊髓束时[5,17,18,50,51,52];认知发育结局与PWML分布位置有关,病灶累及额叶时易发生认知功能障碍[6,17]。
综上,针对PWML的MRI研究,一方面致力于揭示损伤所致脑结构及功能变化的机制,另一方面则为其诊断和预后评估提供影像学依据。尽管如此,现有研究仍存在以下不足:(1)损伤检测方面:PWML多见于早产儿,而该人群大多在矫正到足月时才进行MRI扫描,这样不仅会低估损伤严重程度,还会对预后判断的准确性产生影响。(2)病灶特征方面:PWML位置是判断预后的重要指标之一[6,17],而病灶散在且位置多变,目前尚缺乏对其时空分布特征的深入研究。此外,PWML病灶与其所致脑结构及功能的变化的对应关系还有待进一步阐明。(3)预后评估方面:已有多项研究对PWML患儿的短期发育结局进行了初步探索[5,6,17,18],但如何进行个体化早期预后评估仍是临床亟需解决的核心问题。
因此,PWML病灶特征刻画及个体化早期预后评估仍是未来研究需要努力的方向。由于PWML可随发育进程逐渐消失,故生后早期(2周内)行高分辨率MRI扫描是对病灶及其脑精细结构变化准确评估的重要环节。基于此,可采用多序列成像的同时,融合不同层次的数据分析方法[2,53],进一步对该人群脑皮层结构和网络属性变化特征进行探究。进而基于个体水平建立脑结构变化关键参量与发育结局的潜在映射关系,明确责任病灶,有望实现PWML患儿预后的早期预测。
无。



























