
血氧水平依赖磁共振成像是无创性评价肾内氧代谢功能技术。随着肾脏血氧水平依赖磁共振成像临床研究的持续发展,能监测慢性肾脏病、急性肾损伤、肾小球疾病、肾移植、肾血管疾病以及肾脏肿瘤等多种肾脏疾病的氧合水平变化。笔者综述了关于肾脏血氧水平依赖磁共振成像的基本原理与临床进展。

肾脏氧合改变在多种肾脏疾病发生发展中发挥了重要作用。评估肾脏氧合状态成为肾脏病领域关注的一个新热点。虽然微电极、氧敏感光纤探针能够探测肾脏氧合状态,但是需要在体内进行,具有创伤、出血和感染等并发症。
血氧水平依赖磁共振成像(blood oxygen level-dependent MRI,BOLD-MRI)是一种弛豫率定量手段,提供较高的空间及时间分辨率,具有无创、无辐射和无需注射对比剂等优点[1]。微电极、氧敏光纤探针直接氧检测实验表明,肾脏R2*值和氧分压相关[2]。在临床上,肾脏BOLD-MRI能无创性评价肾脏氧代谢。本文将综述肾脏BOLD-MRI研究进展。
BOLD-MRI是基于体内内源性脱氧血红蛋白实现转化微观磁场和自旋质子去相位,达到改变弛豫参数(R2*=1/T2*)的目的。脱氧血红蛋白导致磁场不稳定、质子去相位速度变快,使得横向弛豫(T2*)短缩、T2*WI信号相对减弱,通过在多个回波时间点捕获和计算信号与回波时间(time of echo,TE)比值的斜率来指示R2*值或T2*值大小。R2*值的升高对应含氧血红蛋白的降低、氧分压减弱、组织缺氧[3]。肾脏髓质的高灌注以及其氧分压梯度使BOLD-MRI可监测到肾脏髓质轻微的脱氧血红蛋白波动,更易实现肾脏氧合评价[4]。
BOLD-MRI成像技术常见有两种,一种是平面回波成像(echo-planar imaging,EPI),另一种是梯度回波成像(gradient echo imaging,GREI)。EPI有卓越的时空分辨率和合理的空间分辨率。EPI容易出现磁敏感度伪影,磁场强度大,局部主磁场同质性低,伪影偏差就更加严重。GREI不易受磁敏感度伪影的干扰,使用范围更广。血液氧合引起的T2*值大小波动和磁场强度是一种线性关系[5]。
临床常用的BOLD-MRI图像分析方法有两种。ROI勾画是在肾脏皮质和髓质相应区域内绘制一个或数个ROI,确定每个扫描层面的皮质和髓质的平均R2*值或T2*值。人工放置在皮质和髓质中的不同大小和不同位置的ROI可能影响R2*值或T2*值的准确性和再现性[6]。十二层面同心对象技术(12 layers concentric objects,TLCO)把肾脏分成12层一样厚度的肾脏层面的半自动程序。所有层面的平均R2*值组合起来就能够绘制成一条具有斜度的曲线。TLCO技术能准确区分肾皮质及肾髓质,人为的影响较小,具有更好的重复性[7]。
肾脏BOLD MRI受许多因素影响,在分析结果时应考虑这些影响因素,更准确地评估肾脏氧合。(1)生理性影响因素:髓质的平均R2*值高于皮质,左肾R2*值略高于右肾,且R2*值随年龄增加而增加[8]。(2)影响组织氧合和血液氧合处于平衡的因素:包括血细胞比容、影响氧气/血红蛋白解离曲线的条件、血流量等[9]。(3)盐水平衡因素:高盐摄入(>15 g/d)导致肾髓质渐进性缺氧,低盐饮食(<5 g/d)可以完全逆转髓质缺氧,水负荷改善肾脏氧合[10]。(4)药物影响:依帕列净、前列地尔、RAS抑制剂和氢氯噻嗪、利尿剂、环孢素改善肾脏氧合,双氯芬酸增加肾脏缺氧[11]。
在慢性肾脏病(chronic kidney disease,CKD)患者中,炎症、氧化应激等很多因素可能导致肾脏组织缺氧。慢性缺氧作为终末期肾病的最终共同途径。BOLD-MRI有助于监测CKD的进展,监测肾功能的变化(表1)[12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25]。BOLD-MRI评估CKD患者肾脏氧和存在争议。Zhou等[13]研究表明CR2*值和MR2*值与肾小球滤过率(glomerular filtration rate,GFR)呈负相关,而Pruijm等[20]研究表明CR2*值和MR2*值与GFR无相关性。研究结果的差异可能与实施BOLD-MRI研究人员之间技术水平的差异有关。早期的一些研究对BOLD-MRI的影响因素缺乏认识,不能使检测患者条件标准、统一化。导致CKD的原因很多,一些文章并没有将导致CKD的原因考虑进去,结果缺乏对比性。肾脏R2*值随年龄的增加而增加,但是在临床应用中BOLD-MRI缺乏不同年龄阶段患者的正常参考值,目前多参考成人标准,结果缺乏对比性。
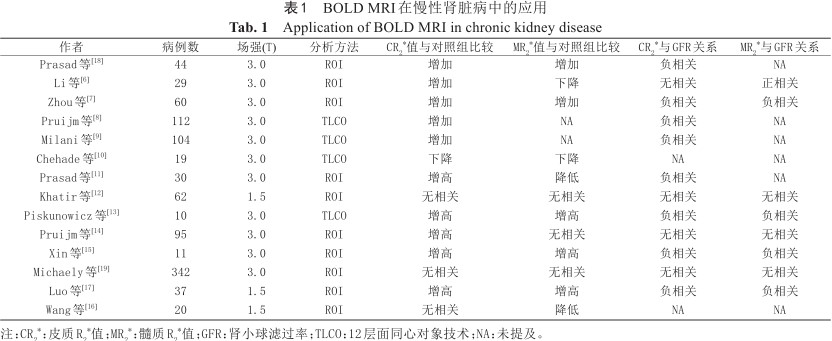
BOLD MRI在慢性肾脏病中的应用
Application of BOLD MRI in chronic kidney disease
BOLD MRI在慢性肾脏病中的应用
Application of BOLD MRI in chronic kidney disease
| 作者 | 病例数 | 场强(T) | 分析方法 | CR2*值与对照组比较 | MR2*值与对照组比较 | CR2*与GFR关系 | MR2*与GFR关系 |
|---|---|---|---|---|---|---|---|
| Prasad等[18] | 44 | 3.0 | ROI | 增加 | 增加 | 负相关 | NA |
| Li等[6] | 29 | 3.0 | ROI | 增加 | 下降 | 无相关 | 正相关 |
| Zhou等[7] | 60 | 3.0 | ROI | 增加 | 增加 | 负相关 | 负相关 |
| Pruijm等[8] | 112 | 3.0 | TLCO | 增加 | NA | 负相关 | NA |
| Milani等[9] | 104 | 3.0 | TLCO | 增加 | NA | 负相关 | NA |
| Chehade等[10] | 19 | 3.0 | TLCO | 下降 | 下降 | NA | NA |
| Prasad等[11] | 30 | 3.0 | ROI | 增高 | 降低 | 负相关 | NA |
| Khatir等[12] | 62 | 1.5 | ROI | 无相关 | 无相关 | 无相关 | 无相关 |
| Piskunowicz等[13] | 10 | 3.0 | TLCO | 增高 | 增高 | 负相关 | 负相关 |
| Pruijm等[14] | 95 | 3.0 | ROI | 增高 | 无相关 | 无相关 | 无相关 |
| Xin等[15] | 11 | 3.0 | ROI | 增高 | 增高 | 负相关 | 负相关 |
| Michaely等[19] | 342 | 3.0 | ROI | 无相关 | 无相关 | 无相关 | 无相关 |
| Luo等[17] | 37 | 1.5 | ROI | 增高 | 增高 | 负相关 | 负相关 |
| Wang等[16] | 20 | 1.5 | ROI | 无相关 | 降低 | NA | NA |
注:CR2*:皮质R2*值;MR2*:髓质R2*值;GFR:肾小球滤过率;TLCO:12层面同心对象技术;NA:未提及。
BOLD-MRI可无创评估缺血性急性肾损伤(acute kidney injury,AKI)患者肾组织含氧量变化。R2*值可以表现出AKI大鼠肾脏皮髓质氧分压的改变,判断缺血程度及氧代谢情况,从而评估肾脏的损害程度[26]。Pohlmann等[27]远端阻塞主动脉,用氧敏感探针和BOLD-MRI获得T2*值同时监测大鼠肾内PaO2。在主动脉阻塞期间,PaO2 下降远大于T2*值下降。在再灌注期间,PaO2即开始恢复,而T2*值在恢复前继续下降。在再灌注后1 h和24 h,BOLD-MRI显示皮质氧合增加,而髓质缺氧持续存在[28]。
BOLD-MRI用于研究对比剂相关肾病。Li等[29]采用BOLD-MRI发现,高渗透性对比剂可以减少肾脏氧合作用,给予碘对比剂后R2*值增加率与急性肾损伤有关。BOLD-MRI评估对比剂急性肾损害大鼠肾功能,肾脏髓质注入对比剂,在半小时内肾脏R2*值急剧上升,但是肾血流量减少较少。肾脏氧合的减少主要是氧消耗增加所致[30]。
急性肾小管坏死患者采用速尿阻断Na+-K+-2Cl-转运体,BOLD-MRI能敏感地检测到轻微的肾小管损伤、肾血管损伤和恢复情况[31]。Tran等[32]采用BOLD-MRI观察脓毒症小鼠,结果显示尽管肾血流减少,仍保持相对正常的氧合水平。这是由于细胞色素氧化酶活性降低,氧消耗减少。BOLD-MRI检测速尿引起肾髓质氧合增加,是因为肾氧消耗减少,肾灌注并未改变。
肾小管间质损害是肾病综合征预后不良的重要因素。氧合减少是导致肾小管间质纤维化的重要原因。Zhang等[33]研究显示,肾病综合征患者髓质R2*值显著降低,髓质R2*值与GFR呈负相关,和肾小管间质损伤评分正相关。
BOLD-MRI评估糖尿病肾病患者氧合状态。糖尿病患者肾脏皮质R2*值水平与血糖和糖化血红蛋白呈正相关,髓质R2*值在糖尿病早期增加。糖尿病肾病患者髓质缺氧相对皮质来说,发生更早、更明显。在糖尿病肾病的进展过程中,可以采用髓质R2*值/皮质R2*值推测糖尿病肾病的进展和预后[34]。
BOLD-MRI评估狼疮性肾炎患者氧合状态。高斯函数模型是应用4个数学公式对R2*值进行拟合,在狼疮性肾炎中,应用拟合后最优化的R2*值来评估狼疮性肾炎患者的氧耐量受损情况[35]。Shi等[36]发现狼疮性肾炎患者的平均肾脏R2*值高于健康志愿者,并且BOLD-MRI可用于鉴别狼疮性肾炎的分型,V型狼疮性肾炎R2*值高于Ⅳ型和Ⅲ型。
肾移植后产生功能障碍的关键作用点有两种,第一种是急性排斥(acute rejection,AR),第二种是急性肾小管坏死(acute tubular necrosis,ATN)。髓质R2*值/皮质R2*值比值可作为区分肾移植急性排斥的标志。肾移植患者R2*值降低有助于预测早期移植物功能降低[36]。在急性排斥组,肾脏髓质R2*值比对照组和急性肾小管坏死组小,髓质R2*值有助于鉴别AR和ATN[37]。
Textort等[38]研究显示,在21例肾图正常的肾脏中,速尿使髓质R2*值下降20%,皮质R2*值下降11.2%。在肾动脉狭窄下游正常肾脏中,R2*值基线升高,给予速尿后,R2*值下降。肾动脉全部闭塞以后,其外的萎缩肾脏就会出现R2*值降低,给予速尿后没有变化。BOLD-MRI用于评价血管闭塞性疾病对肾脏局部组织氧合的影响。
BOLD-MRI不仅探讨肾脏肿瘤的血管分布、血流灌注和氧合作用,还可用于肾脏良性与恶性肿瘤鉴别。透明细胞癌的R2*值比乳头状癌更低,肾脏透明细胞癌的R2*值可作为一种无创生物标志物用于透明细胞癌的分级[39]。
虽然BOLD-MRI对肾氧合状态的评估有明显的优势,但还是存在一些尚需解决的技术难题。目前BOLD-MRI用于检测肾脏氧合国内外缺乏统一技术标准,只有很少的报道涉及了弛豫率正常值及不同场强的相关性[40]。现在的BOLD-MRI研究绝大部分是在经典BOLD软件联合非侵入性血管磁共振成像工具基础上获得R2*值,在肾脏疾病方面的研究鲜有文献报道。
BOLD-MRI结合基于血流的MRI技术以及多参数磁共振技术等多序列MRI技术提高非侵入性检测肾脏内结构、功能和分子变化能力,极大地提高了诊断疾病的能力[41]。定量生理学测量法能联合有创性生理参数、MRI参数和解剖学参数评估肾脏T2*值与肾脏氧合程度的关系[42]。使用软件来分析多参数磁共振的策略,Renal-CAD系统集成了扩散加权成像、BOLD、基因组标记、组织病例标记[43]。国际组织如欧洲科学技术合作行动“慢性肾脏病磁共振成像生物标志物”的目的是协调这一过程,以促进这项技术在不久的将来在临床实践中的应用。
全体作者均声明无利益冲突。



























