
肝细胞癌(hepatocellular carcinoma, HCC)是一种恶性程度高、转移性强、预后差的肿瘤,精准的影像评估有助于HCC患者的诊断及临床治疗决策制订。磁共振弹性成像(magnetic resonance elastography, MRE)为HCC的影像评估提供了一种全新的方式,其能够无创地量化组织力学特性、可重复性强、主观因素影响小、具有较好的临床适用性。MRE通过协助HCC疾病诊断及临床治疗决策制订,可提高患者生活质量及改善预后。本文对MRE在HCC的预测、诊断及鉴别诊断、疗效评估及预后生存预测的研究进展进行综述,分析现阶段MRE技术的优势与不足及未来发展方向,为HCC的诊疗提供有利参考,从而改善HCC患者的预后。

本刊刊出的所有论文不代表本刊编委会的观点,除非特别声明
肝细胞癌(hepatocellular carcinoma, HCC)是一种恶性程度高、转移性强、预后差的肿瘤。据2020年全球肿瘤统计分析结果显示HCC发病率位居全球肿瘤发病率第六位、中国肿瘤发病率第四位,其死亡率位于全球第三位、中国肿瘤死亡率第二位[1, 2]。HCC的早期诊断及治疗评估是关系国计民生的重大战略问题。其中,影像学检查是HCC评估的重要手段,尤其是MRI检查。近年来,随着硬件及软件的进步,MRI已经成为HCC诊断和评估的重要方法之一。扩散加权成像(diffusion-weighted imaging, DWI)、体素内不相干运动扩散加权成像(intravoxel incoherent motion diffusion weighted imaging, IVIM-DWI)、扩散峰度成像(diffusion kurtosis imaging, DKI)、动态对比增强MRI(dynamic contrast enhanced MRI, DCE-MRI)等磁共振肝脏定量评估方法为HCC的诊治评估提供了帮助,但是其在HCC的早期诊断、鉴别诊断、疗效评估和预后预测方面仍然存在不足[3]。
磁共振弹性成像(magnetic resonance elastography, MRE)是一种全新功能MRI技术,为HCC的诊疗评估带来了新的契机,其是一种能够在体内量化组织力学特性的新型非侵入性技术,可直接测量组织硬度[4]。组织硬度是人体组织重要的力学参数和物理参数之一,与生物学特性密切相关,如发育、内环境稳态和疾病发生发展等[5]。目前,MRE已经被广泛用于评估人体不同器官的组织硬度,如头部、胸部、肝脏、前列腺、结直肠等器官[6, 7, 8, 9, 10, 11, 12]。MRE在肝脏疾病的应用最为成熟,尤其是在对肝纤维化的诊断与分级中,可与肝活检相媲美[13, 14]。在强调“精准医学”的今天,对HCC的准确无创的早期诊断、生物学行为预测、疗效评估及预后生存预测极为必要,仅基于影像学表现特征来评估有一定的局限性,主观性较强、可重复性较差,即使是高年资医生评估也存在此问题,而MRE是从生物力学角度无创定量量化组织硬度的一种全新MRI技术,客观性强、可重复性好,MRE为HCC诊疗评估提供了新方法,在HCC诊疗中具有广阔的应用前景。本文着重对MRE在HCC的预测、诊断及鉴别诊断、疗效评估及其在预后生存的研究进展进行综述。
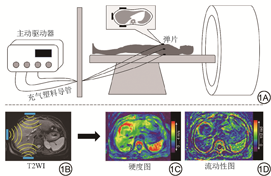
研究表明,随着肿瘤的生长,肿瘤组织及其周围微环境的机械特性会发生变化,且这一改变在疾病的发生、进展和治疗中起着重要的作用[15, 16, 17, 18, 19]。MRE是一种全新功能MRI技术,其能够无创地定量评估人体内组织或器官的力学特性。MRE是通过振动源产生剪切波并通过振动装置传导到所需要检查的部位,再由MRI采集剪切波在组织内的动态传播信息,然后处理剪切波信息,生成显示诸如组织硬度、流动性等机械特性的定量图像。MRE可以用来评估许多器官的硬度,目前最常用于肝脏硬度评估[4,20]。临床肝脏MRE驱动系统的图解及MRE步骤如图1所示。MRE的成像过程可以概括为:(1)产生剪切波;(2)MRI采集剪切波信息;(3)对剪切波进行处理,得到定量图像。在后处理图像中选择感兴趣区(region of interest, ROI)即可得到该区域定量值。

高达70%的HCC患者血清甲胎蛋白(alpha fetoprotein, AFP)水平升高,AFP被认为是HCC的诊断和预后标志物[21]。异常凝血酶原[PIVKA Ⅱ(protein induced by vitamin K absence/antagonist-Ⅱ)、DCP(des-gamma carboxyprothrombin)]、血浆游离微RNA(microRNA, miRNA)和AFP异质体(lens culinaris agglutinin-reactive fraction of AFP, AFP-L3)也可以作为HCC早期诊断标志物,特别是对于血清AFP阴性人群[22, 23, 24, 25, 26]。但是在慢性肝炎和肝纤维化等良性疾病患者中,这些标志物也高度表达,可能产生HCC诊断的假阳性[27]。在预测HCC发生的多项研究表明,MRE优于其他非侵入性检测,如血清标志物[28]、超声瞬时弹性成像(transient elastography, TE)[29]等。Tamaki等[28]研究发现MRE对预测HCC发生的诊断准确率高于血清标志物,MRE、紫藤多花凝集素阳性Mac-2结合蛋白(wisteria floribunda agglutinin-positive mac-2 binding protein, WFA+-M2BP)及肝纤维化4因子(FIB-4)指数的受试者工作特征(receiver operating characteristic, ROC)曲线下面积(area under the curve, AUC)分别为0.743、0.697、0.647,并提出肝脏硬度3.75 kPa是预测HCC发生的最佳临界值。Kumada等[30]的一项纳入了537例慢性肝病患者前瞻性研究中的多变量Cox比例风险模型显示,MRE值>4.5 kPa是唯一与HCC发生独立相关的因素。Ichikawa等[31]回顾性分析了161例慢性肝病患者资料,将患者根据肝脏硬度分为低硬度组(<3 kPa)、中硬度组(3~4.7 kPa)或高硬度组(>4.7 kPa)三组,随访期HCC发生率在高硬度组为46.7%(28/60),中硬度组为26.2%(17/65),低硬度组为5.6%(2/36);高硬度组、中硬度组和低硬度组3年后HCC的发生率分别为45.1%、26.1%和12.4%,差异有统计学意义(P=0.0002),在慢性肝病患者的随访中,应用MRE对肝脏硬度进行纵向观察可以对发生HCC的风险进行危险分层。
上述研究表明,MRE在无创定量预测HCC发生方面具有重要价值,相较于传统血清标志物检测,MRE具有更好的诊断效能,并且MRE能根据肝脏硬度对患者发生HCC的风险进行危险分层,为患者制订更准确的个性化诊疗方案[32],进而改善患者的预后。但是,MRE检查对硬件软件设备要求相对较高,目前普及和推广率尚不足,尚不能作为首选的筛查手段。
Wu等[33]的Meta分析中纳入了1735例患者,MRE对HCC的诊断准确率为100%,敏感度(31%~100%)低于特异度(81%~94%),总的敏感度为64%(95% CI:46%~79%,I2=92.44%),总的特异度为85%(95% CI:82%~88%,I2=67.86%)。结果表明MRE在HCC诊断方面具有良好的敏感度和极好的特异度。MRE可作为HCC的有效诊断工具,为临床治疗方法的选择和预后判断提供有力支持。但这项研究并未对肿瘤大小及分化程度的差异进行比较分析。Thompson等[34]的研究纳入了21例不同大小的HCC,发现根据MRE检测的肿瘤硬度可以区分HCC的分级。这一研究为MRE检测的肿瘤硬度是否能成为HCC病理或分子特征的预测因子奠定了一定的基础。在HCC患者中,Ki-67高水平预示肿瘤的高侵袭性。Hu等[35]将MRE测量的参数利用深度学习联合影像组学模型预测Ki-67表达的AUC为0.90±0.03(95% CI:0.89~0.91)。在独立测试队列中也观察到了同样的结果,AUC为0.83±0.03(95% CI:0.82~0.84)。基于MRE的参数利用深度学习联合影像组学模型可作为评估HCC肿瘤增殖状态的重要方法,表明MRE检测的肿瘤硬度能够预测分子特征。
Shahryari等[9]提出肝脏肿瘤的流动性可能是一种不受非肿瘤性肝组织硬度影响的影像学生物标志物,而非肿瘤性肝组织的硬度通常会受肝纤维化而改变。肿瘤的流动性也与肿瘤转移和侵袭有关[36]。基于新型多频高分辨MRE技术,在77例有病理结果的141个肝脏局灶性病变中,发现反映组织硬度的剪切波速(c)和反映组织流动性的复合剪切模数的相角(φ)在恶性肿瘤中异常高,恶性病变的c和φ显著高于非肿瘤性肝组织(β=3.62,95% CI:2.41~5.73,P<0.001和β=10.64,95% CI:7.02~16.35,P<0.001);φ组AUC为0.95(95% CI:0.92~0.98),c组AUC为0.88(95% CI:0.83~0.94),差异具有统计学意义(P<0.01);c检测恶性病变与肝脏的敏感度为91%(95% CI:86~96),特异度为70%(95% CI:59~81),φ检测恶性病变与肝脏的敏感度为92%(95% CI:87~97),特异度为85%(95% CI:76~93),基于新型多频高分辨MRE技术测量所得参数c和φ可以高精度地区分良恶性肿块,并为肝脏肿瘤提供定量的无创性成像生物标志物。
上述研究表明,MRE可作为HCC的有效诊断工具,为疾病的早期诊断和检出提供更有利的帮助,从而更好地对HCC患者进行管理。当前的研究主要集中在探究MRE对提高HCC诊断准确率的价值,少数研究进一步探究了MRE联合影像组学等方式构建模型预测HCC肿瘤分子标记物。未来可以将MRE与其他定量MRI技术相结合,构建多参数模型有望提高其对HCC诊断及相关肿瘤分子标记物的预测效能。
HCC常见治疗方法包括肝切除术、肝移植术、消融治疗、经动脉化疗栓塞术(transcatheter arterial chemoembolization, TACE)、放射治疗、系统抗肿瘤治疗等多种手段,针对不同分期的肝癌患者选择合理的治疗方法可以使疗效最大化[37]。
在不适合根治性治疗的晚期HCC患者中,通常应用全身或局部治疗,如钇-90微球疗法、TACE和射频消融术(radiofrequency ablation, RFA)等,局部治疗后肿瘤反应的评估对于指导HCC的治疗和预后至关重要。目前用于评估HCC治疗反应的MRI技术主要基于对比增强T1WI(contrast enhanced T1WI, CE-T1WI)上肿瘤残余区域的强化程度差异[38],而MRE是一种基于相位对比度的MRI技术,从生物力学角度来量化组织硬度,评估肿瘤的性质变化,无需使用对比剂,Gordic等[39]在基于CE-T1WI的基础上发现MRE测量的肿瘤硬度与肿瘤的强化(r=0.514,P=0.0001)和坏死(r=-0.540,P=0.0001)显著相关,尤其是在接受钇-90微球疗法治疗的HCC患者中,用MRE测量的肿瘤硬度有可能被用来作为HCC局部治疗反应的一个标志物。Kennedy等[40]在接受钇-90微球疗法治疗的HCC患者中也得到了类似结果,并且发现基线肿瘤硬度、瘤周肝组织硬度和血清AFP是6周后预测HCC患者完全缓解(complete response, CR)的较好指标,但治疗后肿瘤硬度变化的确切机制和时间尺度尚未完全阐明,结合病理相关性和MRE硬度测量的纵向前瞻性研究将是有价值的。
在评估抗肿瘤药物的治疗反应中,目前对HCC的免疫治疗结果尚无影像学预测指标,Qayyum等[41]发现接受抗程序性死亡受体-1(抗PD-1)单抗治疗患者的肿瘤活检中,淋巴细胞数量与MRE测量的肿瘤硬度有显著正相关性(r=0.79,P<0.01),其可能与免疫细胞在肿瘤间质中的改变有关,肿瘤硬度可能是评估治疗反应的一个指标。在口服酪氨酸激酶抑制剂(索拉非尼)的晚期HCC患者中,MRE测量的肝脏硬度是潜在预测生存期和严重肝损伤的生物标志物[42]。MRE已经展示了未来在HCC“精准医学”中的作用,将有助于晚期HCC患者治疗方式的选择,进而改善患者预后。
HCC患者早期肿瘤复发与预后不良有关。以往报道的许多肝癌复发风险预测模型是基于手术切除后获得的病理信息,如微血管侵犯(microvascular invasion, MVI)、肿瘤分化程度、手术切缘状况或免疫组织化学染色结果[43, 44, 45]。在“精准医学”时代,需要在治疗前发现预测早期肿瘤复发的可靠生物标志物,以通过评估个体化的复发风险来确定个性化的治疗策略。
MRE测量的肝脏硬度是慢性肝病患者发生HCC的独立危险因素及治疗后早期复发的预测因子[46, 47, 48]。Park等[49]回顾性分析了98例经病理证实的HCC发现,整个肿瘤的硬度、剔除坏死区后的肿瘤实质硬度和肿瘤大小(≥5 cm)是部分肝切除术后HCC患者复发的预测因素(P均<0.05),但剔除坏死区后的肿瘤实质硬度更能代表HCC的生物侵袭性,可能与胶原沉积增加、异常血流、血管系统改变和肿瘤组织间液压升高有关。
在预测HCC患者术后严重并发症方面,Bae等[29]发现MRE预测术后并发症的AUC显著高于超声TE [0.874(95% CI:0.821~0.916)vs. 0.756(95% CI:0.692~0.813),P=0.020]。在接受肝部分切除术后的HCC患者中,MRE比超声TE更能预测严重的术后并发症。
MVI已被多位研究者证实是肝癌术后复发及转移的独立危险因素[50, 51]。目前大量研究在寻找术前预测MVI的方法,包括利用血清学检查、影像征象等[3,52, 53],然而各研究结果之间存在一定的争议,尚无公认的预测方法。如能在术前无创地预测HCC患者有无MVI,对HCC患者术前的预后评估及临床治疗决策的选择均具有重要意义。最近,一种基于MRE的被称为“滑动界面成像”的剪切应变图的技术已经被用于非侵入性地量化脑肿瘤中肿瘤与瘤周组织的粘连程度,该技术可以识别具有高危手术并发症和手术侵袭性的患者[54, 55, 56]。Li等[57]的研究提供了初步证据,表明基于MRE的剪切应变图有可能作为评估HCC患者肿瘤-肝脏界面状态的生物标志物,并能无创地在术前预测MVI的存在。
MRE是一种新型功能MRI技术,从生物力学角度无创、定量评估组织硬度,可重复性强,主观因素影响小。不仅有助于放射科医生更准确地诊断疾病,还能够为临床医生制订患者个性化诊疗提供帮助。在HCC诊疗方面,MRE技术取得了一定的成果,也表现出了其优势,但仍存在着一些不足和挑战:(1)目前的研究大部分都是单中心、小样本的研究,且随访时间较短,未来需要多中心、大样本的前瞻性研究,并延长随访时间,使试验结果更具有效性;(2)MRE需要配置额外的硬件设备,成本相对较高,检查时间相对较长,因此大规模普及应用尚比较困难;(3)MRE扫描序列、参数及机器设备不同,尚缺乏公认的标准及共识,所获得的图像存在一定的差异性。
尽管MRE技术在HCC诊疗临床应用过程中面临诸多挑战,但随着3D-MRE、多频高分辨MRE、虚拟MRE等新技术的出现[58, 59],使得缩短检查时间及大规模普及成为可能,并有望进一步成为预测肿瘤的分子标记物,为“精准医学”提供更多的信息。将MRE纳入到肝脏MRI检查的常规序列中,特别是肝脏占位性病变的MRI检查,将有望进一步提高对HCC的诊断、鉴别诊断、疗效评估及预后生存预测的价值,为疾病的诊疗提供更有利的工具,从而改善HCC患者的预后。
National Natural Science Foundation of China (No. 82071895).
全体作者均声明无利益冲突。



























