
版权归中华医学会所有。
未经授权,不得转载、摘编本刊文章,不得使用本刊的版式设计。
除非特别声明,本刊刊出的所有文章不代表中华医学会和本刊编委会的观点。
病例1:患者,女,72岁,因"右眼视物不清5 d"就诊。患者主诉无眼红、眼痛等其他症状,否认高血压、糖尿病等其他全身病史。眼科检查:最佳矫正视力(BCVA)为右眼HM/眼前,左眼0.8;双眼眼压14 mmHg(1 mmHg=0.133 kPa)。裂隙灯显微镜检查:双眼晶状体皮质混浊;右眼后极部视网膜下可见约15 PD大暗红色出血灶(见图1A),覆盖黄斑,左眼眼底正常。光学相干断层扫描(OCT)检查示:视网膜组织高度隆起,视网膜下低反射信号,黄斑中心凹厚度1 120 μm(见图1B)。根据以上专科检查及病史,诊断:右眼黄斑下出血;双眼年龄相关性白内障。给予患者右眼玻璃体切割手术,清除内界膜下积血,见视网膜深层仍有6 PD积血,视网膜上血管弓近视盘处动脉血管瘤样扩张,增加诊断右眼视网膜大动脉瘤。术中应用38G针头于视网膜出血灶区穿刺,视网膜下注入组织型纤溶酶原激活剂(Tissue plasminogen activator,t-PA)25 μg(0.025 ml),玻璃体腔填充16% C3F8气体,术中行"白内障超声乳化+人工晶状体植入"术。术后嘱俯卧位。患者于1周后检查发现右眼玻璃体腔气体填充,黄斑区视网膜下积血吸收。患者于术后1个月复查,右眼视力0.05,黄斑中心凹可见约1/2 PD裂孔(见图1C,图1D),给予黄斑裂孔内界膜填塞术,玻璃体腔注入16% C3F8气体,术后俯卧位,1个月后黄斑裂孔愈合(见图1E),右眼BCVA 0.05。
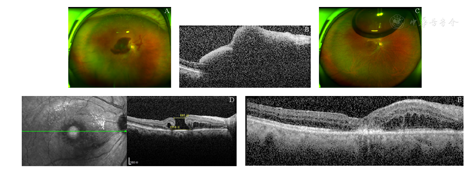

A:右眼黄斑区视网膜下可见约15 PD大小暗红色出血灶;B:OCT示视网膜下大量低反射信号;C:玻璃体切割联合视网膜下注射t-PA术后1个月黄斑下积血全部被吸收,黄斑中心凹见圆形裂孔;D:OCT示术后1个月发现黄斑裂孔形成,直径约885 μm,基底部直径约1 518 μm;E:内界膜填塞术后1个月可见黄斑裂孔愈合
A: There was a dark red subretinal hemorrhage in the macular region of the right eye, about 15 PD in size. B: The OCT showed a lot of subretinal hyporeflexia in the right eye. C: The round macular hole was found after the submacular hemorrhage was absorbed 1 month after vitrectomy combined with subretinal injection of t-PA. D: One month after the operation, OCT showed a macular hole with a diameter of 885 μm and a basal diameter of 1 518 μm in the right eye. E: The macular hole healed 1 month after internal limiting membrane tamponade in the right eye.
病例2:患者,男,74岁,因"左眼突然视物不清1 d"就诊。患者主诉无眼红、眼痛等其他症状,否认高血压、糖尿病等其他全身病史。眼科检查:BCVA为右眼1.0,左眼HM/眼前;眼压为右眼18 mmHg,左眼20 mmHg。裂隙灯显微镜检查示:双眼晶状体皮质混浊;右眼黄斑区见散在玻璃膜疣。左眼黄斑区视网膜下可见约20 PD大暗红色出血灶(见图2A,图2B)。OCT示:右眼见黄斑区视网膜色素上皮层脱离,左眼视网膜高度弧形隆起,视网膜下大量低反射信号,黄斑中心凹厚度1 534 μm(见图2C,图2D)。诊断:右眼黄斑下出血;双眼年龄相关性黄斑变性、年龄相关性白内障。给予患者左眼玻璃体切割术,38G针头于视网膜出血灶区穿刺,视网膜下注入t-PA 25 μg(0.025 ml),玻璃体腔填充16% C3F8气体,术中行"白内障超声乳化+人工晶状体植入"术,术后俯卧位。患者于术后第2天出现玻璃体腔血性混浊,术后20 d后,观察玻璃体腔积血未见明显吸收,给予玻璃体腔灌洗术,术中发现黄斑区视网膜下积血大部分吸收,黄斑中心凹约1/3 PD裂孔(见图2E),剥除裂孔周围内界膜,玻璃体腔注射16% C3F8气体,术后俯卧位,1个月后OCT示黄斑裂孔愈合,黄斑下见少量陈旧性积血(见图2F,图2G),视力达到0.05。
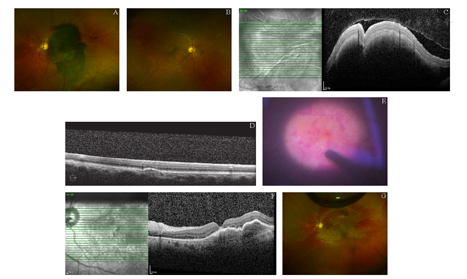

A:视网膜后极部大片视网膜下积血,约20 PD大小;B:右眼黄斑区可见散在玻璃膜疣;C:左眼视网膜弧形隆起,视网膜下大量低反射信号;D:右眼黄斑区见小范围视网膜色素上皮层脱离;E:玻璃体腔灌洗术中发现黄斑区视网膜下积血吸收,黄斑中心凹约1/3 PD裂孔;F:OCT示内界膜填塞术后1个月黄斑裂孔愈合;G:内界膜填塞术后1个月后黄斑区视网膜下积血基本吸收
A: There was a large subretinal hemorrhage in the posterior area of the retina, about 20 PD in size. B: The macular area of the right eye was scattered with drusen. C: In the left eye, the posterior pole of the retina is curved, with subretinal hyporeflexia. D: In the right eye, there is a small area of retinal pigment epithelium detachment in the macular area. E: Subretinal hemorrhage was found in the macular region, and the macular hole with 1/3 PD in size exposed. F: The OCT showed that the macular hole has healed after the surgery for internal limiting membrane tamponade one month later. G: Most of the subretinal hemorrhage in the macular region was absorbed 1 month after the operation.
黄斑下出血是引起视力不可逆性下降的主要原因之一,可有多种原因引起,最常见的有年龄相关性黄斑变性、息肉样脉络膜血管病变(Polypoidal choroidal vasculopathy,PCV)、视网膜大动脉瘤破裂等[1,2,3]。黄斑下出血可以阻隔视网膜神经上皮层间的信号传导,影响视网膜色素上皮与神经上皮之间的物质代谢。大量出血引起的红细胞崩解和血红蛋白变性导致铁离子释放,铁离子介导循环氧化还原反应,产生的自由基对视网膜光感受器细胞产生损伤,血凝块和纤维蛋白的收缩都会对光感受器造成不可逆的损伤[4,5],因此尽早清除黄斑下积血对保存视功能有重要作用。
组织纤溶酶原激活剂可以通过克隆和表达人t-PA基因得到一种丝氨酸类蛋白酶,能特异地激活血栓中的纤溶酶原变成纤溶酶,纤溶酶溶解纤维蛋白致血栓溶解[6]。1996年,Heriot[7]首次提出玻璃体腔内联合注射t-PA和惰性气体治疗黄斑下出血,术后俯卧位,并证明了其有效性。这种联合注射技术的基本原理是t-PA将血凝块溶解为液化状态,随后借助膨胀气体的机械顶压作用将液化的血液移向远离黄斑的区域。研究表明视网膜下注射t-PA联合玻璃体腔注射惰性气体可以很好地清除年龄相关性黄斑变性、PCV、视网膜大动脉瘤、外伤等引起的黄斑下积血,促进视力提高[8,9,10,11]。本研究2例患者黄斑下积血均被有效清除,说明视网膜下注射t-PA能有效清除视网膜下积血。然而t-PA眼内注射的毒性尚未被彻底研究。有实验研究表明,玻璃体内注射大于50 μg剂量对兔和猫视网膜有毒,导致严重的光感受器丢失和视网膜色素上皮(Retinal pigment epithelium,RPE)坏死[12]。Chen等[13]报道了3 d内对黄斑下出血患者进行2次玻璃体内注射50 μg t-PA后出现弥漫性粒状RPE紊乱。而Treumer等[14]在兔眼的视网膜下1次性注射50 μg t-PA后并没有明显不良作用。本研究中的2例患者视网膜下注射25 μg t-PA,均未出现明显视网膜毒性反应。
视网膜下注射t-PA后出现黄斑裂孔的原因不明确,可能由多种原因导致。Sagara等[15]回顾性地分析了56例视网膜大动脉瘤患者,其中7眼(12.5%)出现了黄斑裂孔,7例患者全部并存视网膜下积血,这表明黄斑下出血可能会引起黄斑裂孔。黄斑下出血的动物实验研究表明,兔视网膜在3~7 d内发生严重退化,猫视网膜在7~14 d内发生严重退化[16],纤维蛋白介导的视网膜损伤被认为是视网膜下出血引起的视网膜损伤的重要机制[17]。需要注意的是本研究中2例患者黄斑区积血较多,出血范围均大于15 PD,黄斑中心凹厚度均大于1 000 μm,并且视网膜浅层及深层均有积血,大量的黄斑下积血或者内界膜下积血对黄斑中心凹组织产生很大的顶压力,再加上术中视网膜下注射0.025 ml t-PA药液,进一步增加了视网膜下的压力,机械的顶压力可能导致黄斑裂孔形成[18]。
病例2在术后出现了玻璃体积血,考虑术后早期就已经形成了黄斑裂孔,视网膜下积血通过黄斑裂孔进入玻璃体腔,虽然术中玻璃体腔已经注入16% C3F8惰性气体,术后严格俯卧位,20 d后黄斑裂孔没有愈合,提示视网膜下积血影响黄斑裂孔的愈合。
黄斑裂孔的出现会对视功能造成不可逆损伤,所以在视网膜下注射t-PA治疗黄斑下积血时应该充分考虑到并发黄斑裂孔的可能,做好术前沟通,并应在手术中更加小心轻柔地操作,尽量避免黄斑裂孔的形成。
本研究无任何利益冲突


























