
卵巢癌作为妇科三大恶性肿瘤之一,病死率较高,主要原因是大多数患者发现时都已经进展到Ⅲ或Ⅳ期。卵巢癌主要转移方式为种植转移到盆、腹腔内,其次为局部蔓延、淋巴转移及血行转移,较少转移至乳腺[1]。因此,以乳腺肿物为首发的卵巢癌患者比较罕见,易漏诊、误诊。本研究报告1例卵巢浆液性乳头状癌乳腺转移病例。
卵巢癌作为妇科三大恶性肿瘤之一,病死率较高,主要原因是大多数患者发现时都已经进展到Ⅲ或Ⅳ期。卵巢癌主要转移方式为种植转移到盆、腹腔内,其次为局部蔓延、淋巴转移及血行转移,较少转移至乳腺[1]。因此,以乳腺肿物为首发的卵巢癌患者比较罕见,易漏诊、误诊。本研究报告1例卵巢浆液性乳头状癌乳腺转移病例。
患者女,33岁,因"发现右侧乳腺肿物"于2017-12-07就诊于吉林大学第一医院。既往无冠心病、糖尿病等病史,无肝炎、结核等传染性疾病病史,无吸烟饮酒史,对青霉素类药物过敏,无食物过敏史,15岁初潮,月经规律,G4P1A3L1,姑姑有乳腺癌病史。入院查体:双乳对称,无皮肤红肿及橘皮征,无乳头凹陷及酒窝征。右侧乳腺外上象限距乳头10 cm处可扪及一肿物,大小约1.0 cm×1.0 cm,质地韧,表面光滑,边界尚清,活动性欠佳。双侧腋下及锁骨上未触及肿大淋巴结。辅助检查:乳腺超声及钼靶示右侧乳腺外上象限腺体边缘处可见一囊实性混合回声、稍高密度团块,大小约1.0 cm×1.0 cm,边界清,形态尚规则,其内未见异常钙化点,BI-RADS分级:4级。疑诊乳腺癌。于2017-12-11在全麻下行右侧乳腺区段切除术,术后病理如图1所示,浸润性乳头状癌,伴散在的砂砾样钙化,镜下浸润灶最大径约0.6 cm。免疫组化结果如图2示,切片1:ER(+,70%),PR(+,1%),HER2(-),Ki-67(+,60%),PAX-8(+),WT-1(+),CK5/6(灶状,+),P16(+),Calponin(-),NapsinA(-),P63(-),TTF-1(-),GCDFP-15(-),GATA-3(-),EMA(+);切片2: ER(-),PR(-),HER2(-),KI-67(+,70%),CK-pan(+),P63(-),CA-153(+),E-cadherin(+)。结合免疫组化结果,符合卵巢浆液性乳头状癌转移至乳腺,伴脉管癌栓形成。
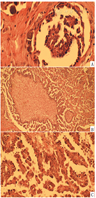

注:A.乳腺浸润性乳头状癌(HE×400);B.卵巢浸润性低级别浆液性癌(HE×100);C.卵巢浸润性低级别浆液性癌(HE×400)。
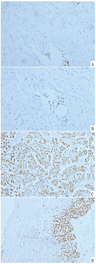

注:A.乳腺组织PAX-8表达阳性;B.乳腺组织WT-1表达阳性;C.卵巢组织PAX-8表达阳性;D.卵巢组织WT-1表达阳性。
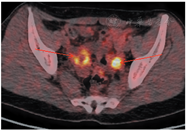
筛查卵巢,回顾病史发现,患者2017-06因偶有左下腹痛行妇科彩超示,左侧卵巢大小35 mm×24 mm;右侧卵巢大小33 mm×28 mm,其内可见31 mm×20 mm囊性无回声,内见分隔,内壁可见絮状低回声,子宫直肠陷窝可见液性暗区,范围43 mm×17 mm,未系统诊治。2017-12-18复查妇科彩超示,双侧卵巢内见囊性为主囊实不均光团回声,见少许血流,右侧卵巢大小为46 mm×34 mm,左侧为46 mm×29 mm。2017-12-20肿瘤标志物检查结果示,CA125为31.01 U/mL,CA153为10.82 U/mL,HE4为74 pmol/L。双侧卵巢肿物性质待查。PET/CT检查示,双侧附件区见囊实团块影,较大约4.4 cm×3.0 cm,伴边缘代谢增高(图3)。于2018-01-05在全麻下行经腹双侧卵巢肿瘤剥除术及卵巢重建术、盆腔粘连松解术、部分大网膜切除术。术后病理示,双侧卵巢病灶浸润性低级别浆液性癌,部分呈表面生长,局部伴微乳头特征,散在砂粒体,大网膜散在少量砂粒体,盆腔有种植(见图1)。免疫组化结果如图2所示,CK7(+),p53(+,<30%),PAX-8(+),ER(+),PR(+),WT-1(+),p16(部分+),Ki-67(+,30%)。腹水脱落细胞学检查找到可疑腺癌细胞。患者卵巢癌乳腺转移诊断明确,再次取右乳区段组织送病理未见残留癌。行遗传性乳腺癌/卵巢癌基因检测,结果为ATM、PALB2两个意义未明基因突变。

于2018-01-16、2018-02-06和2018-02-27分别给予紫杉醇+卡铂静脉化疗3个周期。1个周期化疗后CA125由23.02 U/mL降至11.25 U/mL,全腹CT及乳腺彩超未见肿瘤信号,治疗有效。3个周期化疗后全腹CT见右侧盆腔腹膜局部略增厚,不除外肿瘤播散,为减少肿瘤负荷,2018-03-27在全麻下行"中间性"卵巢肿瘤细胞减灭术(全子宫切除术、双侧附件切除术、大网膜切除术、阑尾切除术、盆腹腔淋巴结切除术)。术后病理如图4所示,左侧卵巢可见少许低级别浆液性癌,右侧卵巢见囊状卵泡,双侧输卵管未见癌,囊性萎缩性子宫内膜,慢性宫颈炎,双侧宫旁组织未见癌,大网膜未见癌,局部见异物(缝线)巨细胞反应,阑尾未见癌,分送各组淋巴结未见癌转移。2018-04-06继续给予原方案化疗1个周期。2018-04-26和2018-05-21分别使用紫杉醇+卡铂方案腹腔灌注2个周期,完成治疗。随访至2019-03未复发,后定期随访。
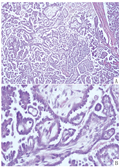

注:A.HE×100;B.HE×400。
乳腺癌是女性中最常见的原发性恶性肿瘤之一,但乳腺外肿瘤转移到乳腺并不常见(0.2%~1.3%),仅约0.03%~0.6%来自原发性卵巢肿瘤,其中以乳腺肿物为首发症状更少见[2]。卵巢癌乳腺转移的组织类型中以浆液性囊腺癌最常见[3],而本例报告为浆液性乳头状癌。
卵巢癌乳腺转移的机制目前尚不清楚,可能从腹腔邻近器官逐步扩散到远端器官,或者根据种子和土壤假说直接通过淋巴和血源途径转移。Signorelli等[4]和Ghezzi等[5]报道,BRCA1, BRCA2或hMSH2突变可能与卵巢癌伴乳腺癌发生有关。然而,这一结论可能仅表明BRCA1、BRCA2和hMSH2突变是不同类型的细胞和器官中的致癌基因,是否在从卵巢癌到乳腺的转移中发挥作用仍有待研究[6]。本研究中这3个基因检测结果均为阴性。另一项研究表明,乳腺转移性病变呈现与癌症传播途径有关[7]。通常血行播散性病变是限制性肿块,并且可以模仿良性肿块。相反,淋巴传播可能导致弥漫性乳房受累,水肿、小梁增厚和皮肤增厚,这可能模仿炎症过程,如乳腺炎或炎性癌。本研究中PET/CT及组织病理均未见淋巴结转移,其他脏器包括腹腔邻近器官未见转移灶,乳腺肿块为限制性,故血行转移可能性大。
原发性卵巢肿瘤的乳腺转移一般在卵巢癌初诊后平均2年出现[8]。本研究以乳腺肿物为首发,除与良性乳腺疾病区分,特别注意与原发性乳腺癌相鉴别,因两者的预后和治疗显著不同。与原发性乳腺癌相比,转移至乳腺的恶性肿瘤常累及单侧(左侧居多)[9],并多位于外上象限,可能与该区域的血液供应较多有关[10]。一般为坚固、界限明显、多结节的肿块,通常是浅表性的,很少与周围组织及皮肤粘连,"橘皮征"不常见[2]。
在影像学方面,乳房外肿瘤转移到乳腺在钼靶X线检查上呈单灶或多灶性的致密肿块,界限清楚,多不伴有钙化,无毛刺边缘、皮肤或乳头凹陷[11]。卵巢浆液性腺癌转移至乳腺较为特殊,镜下常可见沙砾体样钙化灶,这与可见微钙化灶的原发乳腺浸润性乳头状癌难以区分[2]。在MRI中,大多数转移性病变为T2加权序列的中等信号和T1加权序列的低信号,但黑素瘤转移除外,其可能在T1加权序列上显示高信号。在给予顺磁性对比后,通常表现为明显的快速均匀强化,延迟扫描后可见TIC为平台型[12]。
来自原发性卵巢肿瘤的乳腺转移缺乏特征表现,在形态上无法与原发性乳腺癌区分。乳腺和卵巢肿瘤病理形态上可类似,因此,准确的区分需结合免疫组化,其中PAX-8、WT-1、GCDFP-15和GATA-3对于鉴别原发性与转移性乳腺癌的意义较大。研究表明,WT-1在卵巢浆液性癌中阳性率达85%,而原发性乳腺癌中WT-1阳性率仅2%,且通常为灶状弱表达,主要见于粘液亚型[13,14]。PAX-8是卵巢浆液性癌高度敏感和特异的标志物,阳性率达99%,而乳腺癌不表达,且调控WT-1的表达,对卵巢浆液性癌诊断的敏感性和特异性优于WT-1[14,15,16]。GCDFP-15是乳腺癌高度特异的标志物,特异性达96%,但其灵敏度可变,介于23%~73%[17,18]。GATA3在原发性乳腺癌的阳性率介于80%~90%,但缺乏特异性[19],在卵巢癌中阳性率低于10%[20]。另一项研究表明,GATA-3在乳腺癌中的敏感性高于GCDFP-15,故GCDFP-15联合GATA-3也可用于区分原发性乳腺癌与转移性乳腺癌[21]。本研究中PAX-8与WT-1均为阳性,GCDFP-15与GATA3均为阴性,支持卵巢浆液性癌转移到乳腺的诊断。此外,CA125是检测上皮性卵巢癌最具代表性的肿瘤标志物,也是该疾病有效治疗的预测指标,在卵巢浆液性癌中阳性率达99%[22,23],但在一些良性肿瘤患者、3%腹膜炎患者和30%的消化道肿瘤、10%~30%原发性乳腺癌患者中也会显着增加,其低特异性限制了在浆液性卵巢癌诊断中的准确性,但可以作为支持性诊断依据[24,25]。
卵巢浆液性乳头状癌乳腺转移需特别注意与乳腺浸润性微乳头状癌相鉴别。乳腺浸润性微乳头状癌具有特征性组织形态学表现[26,27],肿瘤细胞团呈微(假)乳头状或小腺管状排列,其中的细胞呈"里面朝外"极性颠倒的排列方式;纤细的纤维组织分隔,分隔带无内衬细胞;肿瘤细胞团和和分隔带之间有明显的腔隙,形成主间质分离。免疫表型EMA表达于肿瘤微乳头的边缘和腺管样结构的外侧缘,即独特的"反极"染色现象,而在卵巢浆液性癌中主要表达在肿瘤细胞膜上[28]。该病例乳腺病理未见IMPC特征性组织形态学表现,无EMA"反极"染色现象,可将两者相鉴别。
研究显示,发生乳腺转移的卵巢癌预后较差,生存时间为3~64个月,中位生存时间为8个月[29,30,31]。因此及时正确的诊断、治疗非常重要。治疗上目前尚有争议。大多数认为卵巢癌合并乳腺转移意味着疾病广泛播散,应以全身治疗为主,避免不必要的乳腺手术,乳腺病灶手术仅作为较大肿物的姑息性治疗[2, 32]。有报道表明,对于继发性乳腺癌患者,接受乳腺手术治疗的患者预后显著优于未手术者[28]。还有研究发现,对于卵巢癌转移到乳腺和(或)腋窝的患者,行乳腺和(或)腋窝手术治疗后总生存期较未手术者延长,实践证明术后患者的症状也会显著改善,但应选择合适的患者进行转移灶切除术[33,34,35]。
因此,卵巢癌合并乳腺转移者以全身治疗如化疗、分子靶向治疗为主[36],联合最大限度肿瘤细胞减灭术,使残存肿瘤直径<1.5 cm,同时建议对于有症状的孤立转移到乳腺和(或)腋窝的卵巢癌患者或其他部位转移灶负荷较小的患者,行乳腺和(或)腋窝的转移灶切除术,而对于乳房弥漫受侵或疾病侵袭性强的患者,则避免手术,选择其他姑息性治疗如放射治疗,有望延长患者的生存期[33,34,35]。既往发现,如果受累的腋窝淋巴结>2.5 cm或肿块固定于胸壁或乳腺皮肤广泛溃疡和水肿,应列为手术禁忌[37]。本例患者以乳腺转移灶为首发,故先行乳腺手术,后行新辅助化疗、中间性肿瘤细胞减灭术及辅助化疗,患者症状明显改善。
患者2017-06妇科彩超发现盆腔积液及右侧卵巢不正常,因症状不明显未系统诊治,提醒发现异常需及时就诊,尤其卵巢癌早期症状不明显,不易察觉。对于乳腺肿物,除原发性乳腺肿瘤,尚需警惕其他部位来源肿瘤,如卵巢来源。并建议成年女性要学会自我检查以及掌握卵巢癌与乳腺癌的早期症状,定期做相关检查,以便能早期发现、及时治疗,提高生存率。
Department of Oncology, First Hospital of Jilin University, Changchun 130000, P.R.China
Department of Oncology, First Hospital of Jilin University, Changchun 130000, P.R.China
Department of Oncology, First Hospital of Jilin University, Changchun 130000, P.R.China
Department of Oncology, First Hospital of Jilin University, Changchun 130000, P.R.China
Department of Oncology, First Hospital of Jilin University, Changchun 130000, P.R.China


























