
晚期乳腺癌的治疗主要为姑息性治疗,对于激素受体阳性(HR+)/人类表皮生长因子受体2阴性(HER2-)并且没有内脏危机或威胁生命情况的晚期乳腺癌患者,内分泌治疗是主要的治疗方式。内分泌治疗的目标是延长生存期、改善或维持生活质量并尽可能地延迟化疗的启动。近年来,细胞周期蛋白依赖性激酶4/6(CDK4/6)抑制剂被批准并应用于临床。CDK4/6抑制剂是新型靶向治疗药物,其主要应用于HR+/HER2-乳腺癌患者,使相关内分泌治疗模式有了突破性的进展。目前,被美国食品药品监督管理局批准的CDK4/6抑制剂主要有Palbociclib、Ribociclib和Abemaciclib。与传统的单纯内分泌治疗相比,CDK4/6抑制剂联合内分泌治疗显著延长了乳腺癌患者的无进展生存期,且耐受性良好。主要介绍CDK4/6抑制剂联合内分泌治疗在HR+/HER2-乳腺癌治疗中的临床研究进展,比较不同CDK4/6抑制剂在临床应用中的不良反应。

版权归中华医学会所有。
未经授权,不得转载、摘编本刊文章,不得使用本刊的版式设计。
除非特别声明,本刊刊出的所有文章不代表中华医学会和本刊编委会的观点。
乳腺癌是女性最常见的恶性肿瘤[1]。在每年新发的乳腺癌病例中,大约有3%~10%在诊断时已发生远处转移。在早期乳腺癌患者中,有30%~40%会发展为晚期乳腺癌,而晚期乳腺癌患者的5年生存率仅为20%,中位总生存期为2~3年[2]。按传统的分子分型法,乳腺癌可分为人表皮生长因子受体2(human epidermal growth factor receptor 2,HER2)过表达型(ER/PR-,HER2+)、雌激素受体(estrogen receptor,ER)、孕激素受体(progesterone receptor,PR)、HER2三阴型(ER/PR-,HER2-)、Luminal A型(ER/PR+,HER2-)、Luminal B型(ER/PR+,HER2+)[3]。大约70%的乳腺癌患者的受体表现为ER/PR+[4]。而HR+/HER2-型占所有乳腺癌病例的60%~65%[5]。内分泌治疗是HR+型乳腺癌的有效治疗方式。多年来,由于内分泌治疗的有效性和良好的安全性,辅助进行内分泌治疗是HR+型乳腺癌患者的首选治疗方法[6]。对于HR+型乳腺癌患者,不论其年龄、淋巴结状态如何,或是否应用辅助化疗,术后都应考虑辅助内分泌治疗。用于乳腺癌内分泌治疗的传统药物有3种。一是选择性雌激素受体调节剂(selective estrogen receptor modulators,SERMs),如他莫昔芬(TAM),其可与雌激素竞争性结合雌激素受体从而抑制其功能,主要用于绝经前后女性HR+型乳腺癌的内分泌治疗。二是非甾体类芳香化酶抑制剂(nonsteroidal aromatase inhibitor,NSAI),如来曲唑、阿那曲唑、依西美坦,其通过抑制组织内芳香化酶的活性从而阻止雌激素合成,减少雌激素的生成,主要用于绝经后女性HR+型乳腺癌的内分泌治疗。三是选择性雌激素受体下调剂(selective estrogen receptor degrader,SERDs),如氟维司群(Fulvestrant),其通过下调雌激素受体并减少其作用,主要用于绝经后女性HR+型乳腺癌的内分泌治疗。
内分泌治疗同样也是HR+型晚期乳腺癌的治疗基础,是HR+/HER2-进展期乳腺癌的一线推荐治疗方案[7]。然而,由于基于单药的内分泌治疗会诱导内在耐药性和获得性耐药性,可能会导致癌症复发[8]。因此,迫切需要探索联合治疗策略,以阻止耐药性并改善HR+/HER2-晚期乳腺癌患者的生存获益。细胞周期素依赖激酶4/6(cyclin dependent kinase 4/6,CDK4/6)抑制剂已被美国食品药品管理局(food and drug administration, FDA)批准用于晚期乳腺癌的一线及二线治疗。CDK4/6抑制剂是新型靶向治疗药物,与内分泌单一疗法相比,CDK4/6抑制剂和内分泌治疗联合可显著延长HR+/HER2-型晚期乳腺癌患者的无进展生存期[9]。第一代CDK抑制剂是Flavopiridol(Alvocidib),其是一种泛CDK抑制剂,因其有限的疗效和不可耐受的毒性而导致相关研发被终止[10]。第二代被FDA批准上市的CDK抑制剂包括Palbociclib(帕博西尼),Ribociclib(瑞博西林)和Abemaciclib(玻玛西林),是选择性CDK4/6抑制剂,最近的临床研究结果表明,其与内分泌治疗联合应用,可以显著提高临床疗效[11]。本文中,对上述3种CDK4/6抑制剂的主要临床研究进行综述,并介绍其在临床应用中的主要不良反应。
帕博西尼(Palbociclib),商品名为爱博新(Ibrance),是全球首个上市的CDK4/6抑制剂[12]。2015年2个月,美国FDA根据针对PALOMA-1的二期临床研究结果[13],加速批准Palbociclib联合来曲唑一线治疗绝经后HR+/HER2-型晚期乳腺癌。此后,FDA又根据PALOMA-3的三期临床研究结果[14],批准Palbociclib联合氟维司群治疗绝经后既往内分泌治疗后疾病进展的ER+/HER2-型晚期乳腺癌[15]。
随机二期临床试验PALOMA-1/TRIO-18研究对比了Palbociclib+来曲唑、来曲唑单药在未曾接受过系统治疗的ER+/HER2-型晚期乳腺癌患者中的疗效,Palbociclib+来曲唑组、来曲唑单药组患者的无进展生存期(progression free survival,PFS)分别为20.2和10.2个月[13,16,17]。根据PALOMA-1的研究结果,FDA批准将Palbociclib与来曲唑联合用于ER+/HER2-绝经后晚期乳腺癌的一线治疗[18]。而三期临床试验PALOMA-2证实了Palbociclib+来曲唑的临床疗效,Palbociclib+来曲唑、来曲唑单药组患者的中位PFS分别为24.8和14.5个月(风险比=0.58,P<0.001)[19]。在三期临床试验PALOMA-3中,研究者将患者按2∶1的比例随机分配到氟维司群+Palbociclib组和氟维司群+安慰剂组,患者为绝经状态下经内分泌治疗复发或进展的HR+/HER2-型晚期乳腺癌患者;结果显示,氟维司群+Palbociclib组患者的中位PFS为9.5个月,氟维司群+安慰剂组的中位PFS为4.6个月(风险比=0.46,P<0.001)[20]。2016年2个月,基于三期临床试验PALOMA-3的研究结果,FDA批准扩大适应症,即批准Palbociclib+氟维司群用于二线治疗HR+/HER2-型晚期或转移性乳腺癌患者[21]。在PALOMA-2试验后的另外15个月的后期随访结果显示,与安慰剂+来曲唑相比,Palbociclib+来曲唑持续改善了所有临床相关亚组患者的PFS,并在不减少其使用时间的情况下,大大延迟了化疗的启动时间(Palbociclib+来曲唑、安慰剂+来曲唑组分别为40.4和29.9个月);同时,药物的安全性良好且患者的生活质量得以维持,这为长期影响患者预后提供了早期证据[22]。分析PALOMA-3试验的生存数据发现,在410位对既往内分泌治疗敏感的HR+/HER2-型晚期乳腺癌患者中,Palbociclib+氟维司群组患者的中位总生存期为39.7个月,而安慰剂+氟维司群组的中位总生存期为29.7个月。同时,两组的后续化疗启动时间也存在不同,Palbociclib+氟维司群组患者接受化疗的中位时间为17.6个月,而安慰剂+氟维司群组为8.8个月(P<0.001)。因此,对于既往内分泌治疗敏感的HR+/HER2-型晚期乳腺癌患者,使用Palbociclib+氟维司群治疗比使用安慰剂+氟维司群治疗能获得更长的总生存期[23]。
瑞博西林(Ribociclib),商品名为Kisqali,已获批用于一线联合来曲唑治疗绝经状态下的HR+/HER2-型晚期或转移乳腺癌,及与氟维司群联合用于一、二线治疗绝经后HR+/HER2-型晚期或转移性乳腺癌[24,25]。二期临床试验MONALEESA-1结果表明,来曲唑+Ribociclib在绝经后HR+/HER2-型乳腺癌患者中的耐受性良好,治疗后未观察到3/4级不良事件[26]。MONALEESA-2是一项随机、双盲、安慰剂对照的三期临床试验,其中668例未曾接受过系统治疗的HR+/HER2-型晚期乳腺癌患者按1∶1随机分配,分别接受Ribociclib+来曲唑、安慰剂+来曲唑治疗。其结果表明,联合使用Ribociclib可使患者的PFS从16个月提高到25.3个月,客观缓解率(objective response rate,ORR)分别为52.7%和37.1%(风险比=0.56,P<0.001)[27,28]。基于此项研究成果,2017年3月FDA批准将Ribociclib联合来曲唑用于绝经后HR+/HER2-型晚期或转移性乳腺癌患者的一线治疗[28,29]。在三期临床试验MONALEESA-3中,研究者让绝经后的HR+/HER2-型晚期乳腺癌患者接受Ribociclib+氟维司群、安慰剂+氟维司群治疗,结果显示Ribociclib+氟维司群组患者的中位PFS为20.5个月,安慰剂组为12.8个月,ORR分别为40.9%和28.7%(风险比=0.593,P<0.001)[30]。基于此研究结果,2018年7月FDA批准将Ribociclib联合氟维司群用于绝经后HR+/HER2-型晚期或转移性乳腺癌患者的一线或二线治疗。MONALEESA-7是另一项三期临床试验,用于评估Ribociclib或安慰剂联合内分泌治疗药物(TAM或NSAI)和戈舍瑞林一线治疗绝经前或围绝经期HR+/HER2-型晚期乳腺癌患者的疗效。该研究结果表明,Ribociclib联合组患者的中位PFS为23.8个月,安慰剂联合组为13.0个月,ORR分别为51%和36%(风险比=0.55,P<0.001)[31]。对MONALEESA-3临床试验的总生存结果进行研究后发现,Ribociclib+氟维司群组患者的42个月总生存率为57.8%,安慰剂+氟维司群组为45.9%(P<0.005)。因此,对于HR+/HER2-型晚期乳腺癌患者,用Ribociclib+氟维司群治疗比用安慰剂+氟维司群具有明显的总生存获益[32]。对MONALEESA-7临床试验的总生存结果进行研究后发现,与单独使用内分泌治疗相比,Ribociclib+内分泌治疗可显著延长患者的总生存期,其中Ribociclib+内分泌治疗组患者的42个月总生存率为70.2%,而安慰剂+内分泌治疗组为46.0%(P<0.01)[33]。
玻玛西林(Abemaciclib),商品名为Verzenio,在2017年被FDA批准与氟维司群联合用于内分泌治疗及化疗后进展的HR+/HER2-型晚期或转移性乳腺癌的二线治疗。2018年Abemaciclib获批用于与芳香化酶抑制剂联和用于绝经后HR+/HER2-型晚期或转移性乳腺癌的一线治疗[34]。MONARCH-1是第一项报告Abemaciclib单药活性的二期临床试验,旨在评价Abemaciclib在HR+/HER2-型转移性乳腺癌患者中作为单一治疗药物的有效性和安全性,该研究中,共入组132例既往接受过包括化疗在内的多次治疗失败的晚期乳腺癌患者。其结果显示,Abemaciclib单药组患者的ORR达到19.7%,临床获益率为42.4%,中位PFS为6.0个月,中位总生存期为17.7个月[35]。MONARCH-2是一项三期临床试验,其评价Abemaciclib或安慰剂联合氟维司群用于内分泌治疗后进展的HR+/HER2-型晚期乳腺癌患者二线治疗的效果,入组患者按2∶1随机接受Abemaciclib+氟维司群、安慰剂+氟维司群。结果显示,Abemaciclib+氟维司群组患者的中位PFS为16.4个月,而安慰剂+氟维司群组为9.3个月,ORR分别为48%和21%[36]。基于MONARCH-2的研究结果,2017年9月FDA批准Abemaciclib联合氟维司群用于HR+/HER2-型晚期或转移性乳腺癌患者的二线治疗。根据MONARCH-1的结果,Abemaciclib还被FDA批准作为单药用于治疗HR+/HER2-型晚期或转移性乳腺癌[37]。MONARCH-3是一项随机三期临床试验,其中493例未接受过系统治疗的绝经后HR+/HER2-型晚期乳腺癌患者按2∶1随机接受Abemaciclib+阿那曲唑/来曲唑、安慰剂+阿那曲唑/来曲唑治疗,Abemaciclib联合组患者的中位PFS(28.2个月)明显长于安慰剂联合组(14.8个月),ORR分别为61%和46%[38]。根据MONARCH-3的研究结果,FDA在2018年8月批准Abemaciclib与NSAI联合用于绝经后HR+/HER2-型晚期或转移性乳腺癌的一线治疗[37]。对MONARCH-2临床试验的总体生存结果研究后发现,对于内分泌治疗进展的HR+/HER2-型晚期乳腺癌患者,Abemaciclib+氟维司群组的中位总生存期为46.7个月,而安慰剂+氟维司群组的中位总生存期为37.3个月(P=0.01);Abemaciclib+氟维司群组、安慰剂+氟维司群组患者的中位再次疾病进展时间分别为23.1个月和20.6个月,中位开始化疗时间分别为50.2个月和22.1个月),中位无化疗生存时间分别为25.5个月和18.2个月。可见,Abemaciclib大大延迟了后续化疗的启动时间[39]。
目前,Palbociclib、Ribociclib、Abemaciclib这3种CDK4/6抑制剂均显示出显著的临床疗效[40]。但是,在不同研究的亚组之间回顾性分析并比较这3种CDK4/6抑制剂的区别较为困难,因为每个研究都有不同的设计和不同的患者人群等。相关研究结果显示,这3种CDK4/6抑制剂的患者耐受性良好,但在毒性方面存在一些差异,其中Palbociclib和Ribociclib主要有血液学毒性,Abemaciclib主要有胃肠道毒性而血液学毒性相对不明显[15]。因此,可根据这3种CDK4/6抑制剂的毒副反应特点,依据不同患者的情况选择用药。
中性粒细胞减少是Palbociclib的主要不良反应,常导致不能完成连续给药或需要调整药物剂量[41]。与Palbociclib一样,中性粒细胞减少也是Ribociclib的主要不良反应。但是,Ribociclib导致QT间期延长和肝胆毒性也同样值得注意[42]。Abemaciclib的血液学毒性相对不常见,其不良反应主要为胃肠道不良反应,包括腹泻、恶心、呕吐等,其中腹泻最为常见,可通过给予患者止泻药物或降低药物剂量进行处理[43]。研究结果表明,Palbociclib的常见3/4级不良反应(≥20%)是中性粒细胞减少(54%)、白细胞减少(51%)和淋巴细胞减少(30%)[44];Ribociclib的常见3/4级不良反应(≥20%)是中性粒细胞减少(27%)[44];Abemaciclib的常见3/4级不良反应(≥20%)是白细胞减少(28%)、中性粒细胞减少(27%)和腹泻(20%)[35]。
接下来分析3种CDK4/6抑制剂所呈现不同毒性的内在机制。CDK4对于乳腺肿瘤的发生非常重要,而CDK6在造血干细胞分化中起关键作用[42]。Palbociclib和Ribociclib可抑制CDK4和CDK6,由于CDK6在造血干细胞分化中起关键作用,因此对CDK6的抑制可能导致骨髓抑制(如中性粒细胞减少)并有累积剂量效应。而Abemaciclib对CDK4的抑制作用比对CDK6的抑制作用高14倍,因此其导致的中性粒细胞减少作用,即对骨髓抑制的程度相对较小,与Palbociclib和Ribociclib相比,Abemaciclib对中性粒细胞的抑制作用降低50%[45]。此外,与Palbociclib和Ribociclib仅能抑制CDK4和CDK6不同,Abemaciclib对CDK9也具有额外的抑制作用[46]。这种针对CDK9的作用可以部分解释MONARCH-1临床试验[47]中显示的Abemaciclib单药的临床疗效,以及特定的胃肠道毒性[37]。
多年来,内分泌治疗在临床应用中表现出良好的安全性,CDK4/6抑制剂联合内分泌治疗的常见不良反应与CDK4/6抑制剂单药治疗的常见不良反应基本表现一致。可能会影响Palbociclib、Ribociclib联合内分泌治疗临床应用的常见3/4级不良反应(≥10%)有中性粒细胞减少、白细胞减少。而Abemaciclib联合内分泌治疗在临床应用中常见的3/4级不良反应(≥10%)有中性粒细胞减少和腹泻(表1)。然而,与化疗相比,CDK4/6抑制剂的毒性反应可以通过减少剂量和调整剂量来控制,因此通过常规临床评估,早期、充分地监测药物副作用至关重要,这也是实现成功治疗、最小化副作用和避免治疗中断的关键。
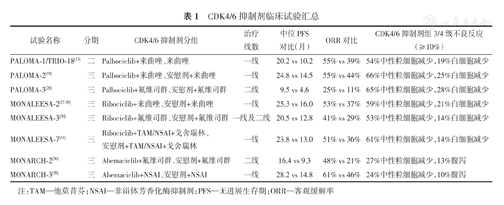
CDK4/6抑制剂临床试验汇总
CDK4/6抑制剂临床试验汇总
| 试验名称 | 分期 | CDK4/6抑制剂分组 | 治疗线数 | 中位PFS对比(月) | ORR对比 | CDK4/6抑制剂组3/4级不良反应(≥10%) |
|---|---|---|---|---|---|---|
| PALOMA-1/TRIO-18[13] | 二 | Palbociclib+来曲唑、来曲唑 | 一线 | 20.2 vs 10.2 | 55% vs 39% | 54%中性粒细胞减少,19%白细胞减少 |
| PALOMA-2[19] | 三 | Palbociclib+来曲唑、安慰剂+来曲唑 | 一线 | 24.8 vs 14.5 | 55% vs 44% | 66%中性粒细胞减少,25%白细胞减少 |
| PALOMA-3[20] | 三 | Palbociclib+氟维司群、安慰剂+氟维司群 | 二线 | 9.5 vs 4.6 | 25% vs 11% | 65%中性粒细胞减少,28%白细胞减少 |
| MONALEESA-2[27,28] | 三 | Ribociclib+来曲唑、安慰剂+来曲唑 | 一线 | 25.3 vs 16.0 | 53% vs 37% | 59%中性粒细胞减少,21%白细胞减少 |
| MONALEESA-3[30] | 三 | Ribociclib+氟维司群、安慰剂+氟维司群 | 一线及二线 | 20.5 vs 12.8 | 41% vs 29% | 53%中性粒细胞减少,14%白细胞减少 |
| MONALEESA-7[31] | 三 | Ribociclib+TAM/NSAI+戈舍瑞林、安慰剂+TAM/NSAI+戈舍瑞林 | 一线 | 23.8 vs 13.0 | 51% vs 36% | 61%中性粒细胞减少,14%白细胞减少 |
| MONARCH-2[36] | 三 | Abemaciclib+氟维司群、安慰剂+氟维司群 | 二线 | 16.4 vs 9.3 | 48% vs 21% | 27%中性粒细胞减少,13%腹泻 |
| MONARCH-3[38] | 三 | Abemaciclib+NSAI、安慰剂+NSAI | 一线 | 28.2 vs 14.8 | 61% vs 46% | 24%中性粒细胞减少,10%腹泻 |
注:TAM—他莫昔芬;NSAI—非甾体芳香化酶抑制剂;PFS—无进展生存期;ORR—客观缓解率
综上所述,由于CDK4/6在乳腺癌的发生、发展中的重要作用,CDK4/6抑制剂彻底改变了HR+/HER2-型乳腺癌的治疗模式。CDK4/6抑制剂联合内分泌治疗已成为HR+/HER2-型乳腺癌患者的新治疗策略并显著改善了患者的预后。Palbociclib、Ribociclib、Abemaciclib均在美国获批与NSAI或氟维司群联合用于HR+/HER2-型乳腺癌的一线或二线治疗。Palbociclib和Ribociclib的最常见不良事件是血液学毒性,尤其是中性粒细胞减少,而腹泻等胃肠道不良反应更常见于Abemaciclib。虽然大量的临床研究结果已证实CDK4/6抑制剂的显著疗效,然而也有部分患者表现出耐药,关于耐药机制的研究目前还处于起步阶段,其具体机制和疾病进展后CDK4/6抑制剂的作用,还需要继续研究与探讨。随着CDK4/6抑制剂在临床上的广泛应用,需要更精确的研究来指导乳腺癌的个体化治疗以及CDK4/6抑制剂与其他药物的联合,以进一步改善晚期乳腺癌患者的生存获益。



























