
他汀类药物(简称他汀)是目前最为常用的药物之一,而他汀的不良反应尤其是肝毒性也越来越引起人们的关注,但目前关于不同他汀对肝功能影响的评价不一。
系统评价不同他汀对肝功能的影响。
计算机检索Cochrane Library、PubMed、EMBase、Medline,检索干预措施为他汀、不良反应涉及肝功能异常的随机对照试验(RCTs),检索时限为建库至2020-09-10。由2位研究者进行筛选文献、提取资料、评价纳入文献的偏倚风险,采用Stata 14软件进行网状Meta分析。
共纳入46篇RCTs,54 499例患者,包含6种药物:阿托伐他汀(21篇)、瑞舒伐他汀(10篇)、辛伐他汀(10篇)、普伐他汀(7篇)、洛伐他汀(4篇)、匹伐他汀(3篇)。网状Meta分析结果显示,阿托伐他汀肝功能异常发生率高于瑞舒伐他汀、普伐他汀,阿托伐他汀、瑞舒伐他汀肝功能异常发生率高于安慰剂(P<0.05)。累积排序曲线(SUCRA)对不同他汀的排序结果为安慰剂(77.2%)>匹伐他汀(70.9%)>普伐他汀(64.1%)>辛伐他汀(60.7%)>瑞舒伐他汀(40.4%)>洛伐他汀(31.6%)>阿托伐他汀(5.1%)。通过细胞色素P450氧化酶(CYP450酶)进行代谢的他汀肝功能异常发生率高于安慰剂(P<0.05)。SUCRA对不同代谢途径他汀的排序结果为安慰剂(95.6%)>不通过CYP450酶进行代谢的他汀(51.9%)>通过CYP450酶进行代谢的他汀(2.6%)。脂溶性他汀肝功能异常发生率高于安慰剂(P<0.05)。SUCRA对不同性质他汀的排序结果为安慰剂(97.4%)>水溶性他汀(48.7%)>脂溶性他汀(3.9%)。高强度阿托伐他汀肝功能异常发生率高于中强度匹伐他汀、中强度普伐他汀、中强度辛伐他汀、高强度瑞舒伐他汀、中强度瑞舒伐他汀,高强度阿托伐他汀、高强度瑞舒伐他汀肝功能异常发生率高于安慰剂(P<0.05)。SUCRA对不同剂量他汀的排序结果为中强度瑞舒伐他汀(76.4%)>中强度匹伐他汀(71.6%)>安慰剂(69.1%)>中强度辛伐他汀(67.5%)>中强度普伐他汀(55.1%)>低强度普伐他汀(41.7%)>高强度瑞舒伐他汀(40.9%)>中强度阿托伐他汀(37.9%)>低强度洛伐他汀(31.0%)>高强度阿托伐他汀(8.8%)。
脂溶性他汀、通过CYP450酶进行代谢的他汀、高剂量他汀肝功能异常发生率较高。本研究纳入文献较多且总体质量尚可,对临床用药具有一定的指导意义,但部分文献质量不高,仍需开展更多高质量的RCTs对本研究结果进行验证。

本刊2021年版权归中国全科医学杂志社所有
未经编辑部许可,不得任意转载和摘编
本刊所发表作品仅为作者观点,并不代表编委会和编辑部意见
如有印装质量问题请向本刊发行部调换
他汀类药物(简称他汀)是目前最为常用的药物之一[1,2],具有降脂、改善内皮功能、抗炎、免疫调节、抗血栓等多种作用[3],可以有效地降低心脑血管疾病发病率及死亡率[4,5,6,7,8,9,10]。但许多患者因他汀不良反应对其依从性较差[11,12],且随着他汀使用者增加及其常与不同药物联用,引起潜在不良反应增加,限制了他汀的使用。其中药物性肝损伤占他汀所致不良反应的57%,在服用他汀的总人群中发病率为1.2/10万人[13,14]。因此美国食品药品监督管理局(FDA)建议,应在服用他汀之前在基线评估肝功能[15],同时,临床医生应了解不同他汀对肝功能的影响,了解不同类型(如不同代谢途径、亲脂性)他汀和不同剂量他汀对肝功能的影响,才能尽可能在获得他汀最大治疗效果的同时避免对肝功能的损害。
目前多数随机对照试验(randomized clinical trial,RCTs)为双臂安慰剂对照试验,普通Meta分析仅能实现药物两两之间的比较[16],不能简单明了的显示出各种他汀之间的比较结果,不利于他汀的选择。既往也没有网状Meta分析评价不同代谢途径、不同亲脂性、不同剂量他汀对肝功能的影响。本研究计划收集他汀治疗且不良反应涉及肝功能异常的RCTs,采用网状Meta分析对这些RCTs进行分析,以期获得不同他汀对肝功能影响的比较结果。本研究遵照《NMA优先报告条目-The PRISMA扩展申明》进行报告[17]。
RCTs。
(1)本研究为首次多方面比较和评价不同他汀类药物(简称他汀)对肝功能影响的网络Meta分析,且纳入研究及例数较多。(2)本研究纳入的随机对照试验(RCTs)为随机分组,且排除了开放性标签的研究,可减少选择偏倚并提高结果的可靠性。(3)使用累积排序曲线(SUCRA)检测7种干预措施之间的细微差异。(4)不一致性检验没有显示实质性的不一致。
(1)纳入人群包括全部使用他汀的患者,患者自身病情、联合用药等混杂因素较多,可能影响研究结果。(2)纳入的一些RCTs的随访时间较短,可能不能很好地体现他汀对肝功能的长期影响。(3)查找文献时找到一篇关于氟伐他汀合格的文献,但因纳入后95%CI较大,故舍弃,未能进行氟伐他汀与安慰剂及其他他汀的比较。
使用他汀的患者及其对照组,不限制患者所患疾病。
他汀与安慰剂或另一种他汀进行比较。他汀包括:辛伐他汀、洛伐他汀、氟伐他汀、阿托伐他汀、匹伐他汀、普伐他汀和瑞舒伐他汀。
肝功能异常:血清丙氨酸氨基转移酶(ALT)和/或血清天冬氨酸氨基转移酶(AST)≥2倍正常上线值(ULN)、总胆红素(Bilirubin)和/或碱性磷酸酶(ALP)≥2ULN[18]。
(1)会议摘要、信件、病例报告及综述;(2)重复发表文献;(3)动物实验;(4)开放性标签RCTs;(5)试验过程中使用非他汀类降脂药物或影响肝功能的药物。
计算机检索Cochrane Library、PubMed、EMBase、Medline,检索干预措施为他汀、不良反应涉及肝功能异常的RCTs,检索时限为建库至2020-09-10;手工检索相关参考文献以补充文献。英文检索词为:statin、atorvastatin、rosuvastatin、simvastatin、pravastatin、lovastatin、fluvastatin、pitavastatin、bilirubin、aspartate aminotransferase(AST)、alanine aminotransferase(ALT)、alkaline phosphatase(ALP)、cholinesterase(ChE)、lactate dehydrogenase(LD/LDH)、hepatotoxicity、liver function、liver injury、liver damage、liver toxicity、random等。
由2名研究者进行文献筛选及资料提取,排除重复文献后首先阅读题目及摘要排除明显不符合要求的文献,对剩余文献进行全文阅读,明确是否符合纳入标准,如有必要,通过各种联系方式(电话、邮箱)联系原作者获取所需信息,对需要纳入的文献由2名研究者交叉核对是否符合标准,对不确定是否纳入的文献由第3名研究者仔细阅读后决定。
纳入的文献资料包括:(1)基本信息:文章题目、第一作者、发表时间、国家/地区等;(2)研究特征:试验组及对照组的干预措施、研究对象例数、年龄、随访时间;(3)文献偏倚风险评价所需的关键信息;(4)所需的结局指标:试验组及对照组肝功能异常人数。
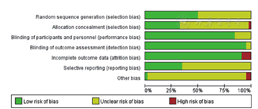
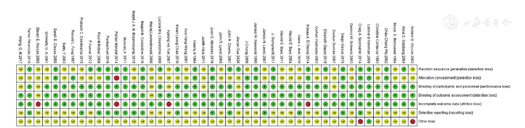
RCTs的研究质量由2位研究者使用Cochrane系统评价员手册5.1推荐的评估偏倚风险的工具进行评估[19,20],使用RevMan 5.3绘制偏倚风险相关图表。该工具包括随机方法、分配隐藏、盲法(研究者和受试者)、盲法(结局测量者)、结局数据完整、选择性报告结果和其他偏倚来源。每个方面可进一步归类为低风险、高风险或不明确风险。
本研究采用Stata 14软件进行数据的分析比较,选用相对危险度(RR)及95%可信区间(95%CI)作为二分类变量分析统计量。使用不一致性检验检测直接证据和间接证据之间是否存在不一致,若P>0.05,则认为不存在总体不一致性,反之则存在总体不一致性;若假设检验比值比(ROR)的95%CI包含1,则认为不存在局部不一致性,反之则存在局部不一致性[21]。通过预测区间图判断异质性,若95%CI和95%预测区间(95%PrI)均包含1或均不包含1则认为无统计学异质性,反之则存在统计学异质性。通过计算累积排序曲线(SUCRA)下的面积评价不同他汀对肝功能的影响,SUCRA值越高,则他汀对肝功能的影响越小,成为最优的可能性越大[22]。以P<0.05为差异有统计学意义。
经检索,共纳入46篇文献[23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68],54 499例患者,其中包含6种药物:阿托伐他汀(21篇文献[23,25,30,32,34,37,39,40,44,45,46,47,49,51,56,58,59,60,61,62,65])、瑞舒伐他汀(10篇文献[35,36,38,41,42,43,49,56,57,60])、辛伐他汀(10篇文献[24,28,29,50,52,54,55,58,59,66])、普伐他汀(7篇文献[27,30,33,48,51,67,68])、洛伐他汀(4篇文献[26,53,63,64])、匹伐他汀(3篇文献[31,33,39])。文献筛选流程见图1,文献基线特征见表1,文献偏倚风险评价见图2,图3。
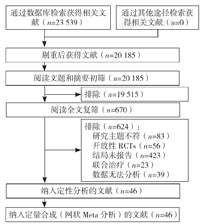

注:RCTs=随机对照试验


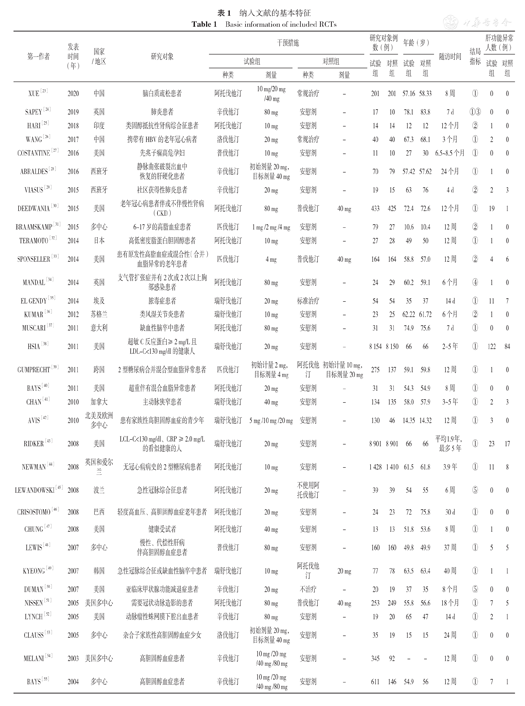
纳入文献的基本特征
Basic information of included RCTs
纳入文献的基本特征
Basic information of included RCTs
| 第一作者 | 发表时间(年) | 国家/地区 | 研究对象 | 干预措施 | 研究对象例数(例) | 年龄(岁) | 随访时间 | 结局指标 | 肝功能异常人数(例) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 试验组 | 对照组 | 试验组 | 对照组 | 试验组 | 对照组 | 试验组 | 对照组 | ||||||||
| 种类 | 剂量 | 种类 | 剂量 | ||||||||||||
| XUE[23] | 2020 | 中国 | 脑白质疏松患者 | 阿托伐他汀 | 10 mg/20 mg /40 mg | 常规治疗 | - | 201 | 201 | 57.16 | 58.33 | 8周 | ① | 0 | 0 |
| SAPEY[24] | 2019 | 英国 | 肺炎患者 | 辛伐他汀 | 80 mg | 安慰剂 | - | 17 | 10 | 78.1 | 83.8 | 7 d | ①③ | 0 | 0 |
| HARI[25] | 2018 | 印度 | 类固醇抵抗性肾病综合征患者 | 阿托伐他汀 | 10 mg | 安慰剂 | - | 14 | 14 | 12 | 12 | 12个月 | ② | 1 | 0 |
| WANG[26] | 2017 | 中国 | 携带有HBV的老年冠心病者 | 洛伐他汀 | 20 mg | 常规治疗 | - | 40 | 40 | 67.3 | 68.1 | 3个月 | ① | 2 | 0 |
| COSTANTINE[27] | 2016 | 美国 | 先兆子痫高危孕妇 | 普伐他汀 | 10 mg | 安慰剂 | - | 11 | 10 | 27 | 30 | 6.5~8.5个月 | ① | 0 | 0 |
| ABRALDES[28] | 2016 | 西班牙 | 静脉曲张破裂出血中恢复的肝硬化患者 | 辛伐他汀 | 初始剂量20 mg,目标剂量40 mg | 安慰剂 | - | 70 | 79 | 57.42 | 57.62 | 24个月 | ① | 1 | 0 |
| VIASUS[29] | 2015 | 西班牙 | 社区获得性肺炎患者 | 辛伐他汀 | 20 mg | 安慰剂 | - | 19 | 15 | 63 | 76 | 4 d | ② | 2 | 3 |
| DEEDWANIA[30] | 2015 | 美国 | 老年冠心病患者伴或不伴慢性肾病(CKD) | 阿托伐他汀 | 80 mg | 普伐他汀 | 40 mg | 433 | 425 | 72.4 | 72.6 | 12个月 | ① | 19 | 1 |
| BRAAMSKAMP[31] | 2015 | 多中心 | 6~17岁的高脂血症患者 | 匹伐他汀 | 1 mg /2 mg /4 mg | 安慰剂 | - | 79 | 27 | 10.6 | 10.4 | 12周 | ② | 1 | 0 |
| TERAMOTO[32] | 2014 | 日本 | 高低密度脂蛋白胆固醇患者 | 阿托伐他汀 | 10 mg | 安慰剂 | - | 27 | 28 | 49 | 50 | 12周 | ① | 1 | 0 |
| SPONSELLER[33] | 2014 | 美国 | 患有原发性高脂血症或混合性(合并)血脂异常的老年患者 | 匹伐他汀 | 4 mg | 普伐他汀 | 40 mg | 164 | 164 | 58.8 | 57.0 | 12周 | ② | 4 | 6 |
| MANDAL[34] | 2014 | 英国 | 支气管扩张症并有2次或2次以上胸部感染患者 | 阿托伐他汀 | 80 mg | 安慰剂 | - | 24 | 29 | 60.2 | 59.1 | 6个月 | ④ | 1 | 0 |
| EL GENDY[35] | 2014 | 埃及 | 脓毒症患者 | 瑞舒伐他汀 | 20 mg | 标准治疗 | - | 54 | 54 | 35 | 37 | 14 d | ① | 11 | 7 |
| KUMAR[36] | 2012 | 苏格兰 | 类风湿关节炎患者 | 瑞舒伐他汀 | 10 mg | 安慰剂 | - | 23 | 25 | 62.22 | 61.72 | 6个月 | ② | 1 | 0 |
| MUSCARI[37] | 2011 | 意大利 | 缺血性脑卒中患者 | 阿托伐他汀 | 80 mg | 安慰剂 | - | 31 | 31 | 74.9 | 75.6 | 7 d | ① | 0 | 0 |
| HSIA[38] | 2011 | 美国 | 超敏C反应蛋白≥2 mg/L且LDL-C<130 mg/dl的健康人 | 瑞舒伐他汀 | 20 mg | 安慰剂 | - | 8 154 | 8 150 | 66 | 66 | 2~5年 | ① | 122 | 84 |
| GUMPRECHT[39] | 2011 | 跨国 | 2型糖尿病合并混合型血脂异常患者 | 匹伐他汀 | 初始计量2 mg,目标剂量4 mg | 阿托伐他汀 | 初始计量10 mg,目标剂量20 mg | 275 | 137 | 59.1 | 59.8 | 12周 | ① | 1 | 0 |
| BAYS[40] | 2011 | 美国 | 超重伴有混合血脂异常患者 | 阿托伐他汀 | 20 mg | 安慰剂 | - | 31 | 31 | 54.3 | 54.9 | 8周 | ① | 0 | 0 |
| CHAN[41] | 2010 | 加拿大 | 主动脉狭窄患者 | 瑞舒伐他汀 | 40 mg | 安慰剂 | - | 134 | 135 | 58.0 | 57.9 | 3~5年 | ① | 2 | 3 |
| AVIS[42] | 2010 | 北美及欧洲多中心 | 患有家族性高胆固醇血症的青少年 | 瑞舒伐他汀 | 5 mg /10 mg /20 mg | 安慰剂 | - | 130 | 46 | 14.35 | 14.32 | 12周 | ① | 3 | 0 |
| RIDKER[43] | 2008 | 美国 | LCL-C<130 mg/dl、CRP≥2.0 mg/L的看似健康的人 | 瑞舒伐他汀 | 20 mg | 安慰剂 | - | 8 901 | 8 901 | 66 | 66 | 平均1.9年,最多5年 | ① | 23 | 17 |
| NEWMAN[44] | 2008 | 英国和爱尔兰 | 无冠心病病史的2型糖尿病患者 | 阿托伐他汀 | 10 mg | 安慰剂 | - | 1 428 | 1 410 | 61.5 | 61.8 | 3.9年 | ① | 11 | 8 |
| LEWANDOWSKI[45] | 2008 | 波兰 | 急性冠脉综合征患者 | 阿托伐他汀 | 20 mg | 不使用阿托伐他汀 | - | 39 | 39 | 54 | 55 | 6周 | ⑤ | 0 | 0 |
| CRISOSTOMO[46] | 2008 | 巴西 | 轻度高血压、高胆固醇血症老年患者 | 阿托伐他汀 | 20 mg | 安慰剂 | - | 24 | 23 | 72 | 75.8 | 30 d | ① | 0 | 0 |
| CHUNG[47] | 2008 | 美国 | 健康受试者 | 阿托伐他汀 | 40 mg | 安慰剂 | - | 13 | 13 | 51.8 | 53.6 | 8周 | ① | 1 | 0 |
| LEWIS[48] | 2007 | 多中心 | 慢性、代偿性肝病伴高胆固醇血症患者 | 普伐他汀 | 80 mg | 安慰剂 | - | 160 | 160 | 49.8 | 49.9 | 37周 | ① | 5 | 5 |
| KYEONG[49] | 2007 | 韩国 | 急性冠脉综合征或缺血性脑卒中患者 | 瑞舒伐他汀 | 10 mg | 阿托伐他汀 | 20 mg | 77 | 78 | 63.5 | 63.4 | 40周 | ① | 1 | 1 |
| DUMAN[50] | 2007 | 美国 | 亚临床甲状腺功能减退症患者 | 辛伐他汀 | 20 mg | 不治疗 | - | 20 | 19 | 37 | 35 | 8个月 | ⑤ | 0 | 0 |
| NISSEN[51] | 2005 | 美国多中心 | 需要冠状动脉造影的患者 | 阿托伐他汀 | 80 mg | 普伐他汀 | 40 mg | 253 | 249 | 55.8 | 56.6 | 18个月 | ① | 7 | 5 |
| LYNCH[52] | 2005 | 美国 | 动脉瘤性蛛网膜下腔出血患者 | 辛伐他汀 | 80 mg | 安慰剂 | - | 19 | 20 | 65 | 47 | 14 d | ① | 2 | 1 |
| CLAUSS[53] | 2005 | 多中心 | 杂合子家族性高胆固醇血症少女 | 洛伐他汀 | 初始剂量20 mg,目标剂量40 mg | 安慰剂 | - | 35 | 19 | 15 | 15 | 24周 | ① | 0 | 0 |
| MELANI[54] | 2003 | 美国多中心 | 高胆固醇血症患者 | 辛伐他汀 | 10 mg /20 mg /40 mg /80 mg | 安慰剂 | - | 345 | 92 | - | - | 12周 | ① | 0 | 0 |
| BAYS[55] | 2004 | 多中心 | 高胆固醇血症患者 | 辛伐他汀 | 10 mg /20 mg /40 mg /80 mg | 安慰剂 | - | 611 | 146 | 54.9 | 56 | 12周 | ① | 7 | 1 |
| SCHNECK[56] | 2003 | 美国及加拿大多中心 | 高胆固醇血症患者 | 瑞舒伐他汀 | 5 mg /10 mg /20 mg /40 mg /80 mg | 阿托伐他汀 | 10 mg /20 mg /40 mg /80 mg | 209 | 165 | 56.93 | 56.46 | 6周 | ① | 1 | 2 |
| SAITO[57] | 2003 | 日本 | 空腹160 mg/dl<LDL-C<220 mg/dl、三酰甘油<300 mg/dl患者 | 瑞舒伐他汀 | 1 mg /2.5 mg /5 mg /10 mg /20 mg /40 mg | 安慰剂 | - | 97 | 15 | 54.75 | 58.50 | 6周 | ① | 0 | 0 |
| KADIKOYLU[58] | 2003 | 土耳其 | 无冠心病的原发性高胆固醇血症患者 | 阿托伐他汀 | 初始剂量10 mg,未达目标者增为20 mg | 辛伐他汀 | 初始剂量10 mg,未达目标者增为20 mg | 35 | 26 | 53 | 54 | 24周 | ① | 0 | 0 |
| WU[59] | 2002 | 亚洲多中心 | 160 mg/dl<LDL-C<250 mg/dl、三酰甘油<400 mg/dl患者 | 阿托伐他汀 | 初始剂量10 mg,目标剂量20 mg | 辛伐他汀 | 初始剂量10 mg,目标剂量20 mg | 76 | 75 | 54.7 | 55.7 | 16周 | ① | 0 | 1 |
| OLSSON[60] | 2002 | 多中心 | 高胆固醇血症患者 | 瑞舒伐他汀 | 5 mg /10 mg | 阿托伐他汀 | 10 mg | 268 | 140 | 57.05 | 58.20 | 52周 | ① | 0 | 2 |
| WANG[61] | 2001 | 中国台湾 | 160 mg/dl<LDL-C<250 mg/dl患者 | 阿托伐他汀 | 10 mg | 安慰剂 | - | 26 | 28 | 66.8 | 65.4 | 8周 | ① | 0 | 0 |
| SCHWARTZ[62] | 2001 | 多国多中心 | 18岁及以上不稳定型心绞痛或非q波急性心肌梗死患者 | 阿托伐他汀 | 80 mg | 安慰剂 | - | 1 538 | 1 548 | 65 | 65 | 16周 | ① | 38 | 9 |
| DOWNS[63] | 2001 | 美国 | 一般健康的中老年人 | 洛伐他汀 | 初始剂量20 mg,目标剂量20~40 mg | 安慰剂 | - | 3 242 | 3 248 | 58 | 58 | 5.2年 | ① | 18 | 11 |
| FONG[64] | 1997 | 美国 | 原发性高胆固醇血症的非裔美国人 | 洛伐他汀 | 20 mg | 安慰剂 | - | 22 | 19 | 53.3 | 51.3 | 10周 | ① | 0 | 0 |
| NAWROCKI[65] | 1995 | 加拿大美国多中心 | 原发性高胆固醇血症患者 | 阿托伐他汀 | 2.5 mg/5 mg/10 mg/20 mg/40 mg/80 mg | 安慰剂 | - | 67 | 12 | 56 | 54 | 6周 | ① | 1 | 0 |
| KEECH[66] | 1994 | 英国 | 冠心病风险增加的人 | 辛伐他汀 | 20 mg/40 mg | 安慰剂 | - | 414 | 207 | 63.4 | 63.7 | 中位3~4年 | ① | 17 | 8 |
| BEHOUNEK[67] | 1994 | 多国 | 非胰岛素依赖型糖尿病和高胆固醇血症患者 | 普伐他汀 | 10 mg | 安慰剂 | - | 167 | 158 | 58.3 | 58.3 | 16周 | ① | 1 | 0 |
| CONTACOS[68] | 1993 | 澳大利亚 | 混合性高脂血症患者 | 普伐他汀 | 40 mg | 安慰剂 | - | 10 | 11 | 52 | 64 | 6周 | ② | 0 | 0 |
注:①=ALT和/或AST≥3ULN,②=ALT和/或AST≥2ULN,③=总胆红素≥2ULN,④=ALT和/或AST≥5ULN,⑤=ALT和/或AST均正常;-为无此数值;ALT=丙氨酸氨基转移酶,AST=天冬氨酸氨基转移酶,ULN=正常上限值,LDL-C=低密度脂蛋白胆固醇,CRP=C反应蛋白
各比较结果的干预措施的网络关系见图4。
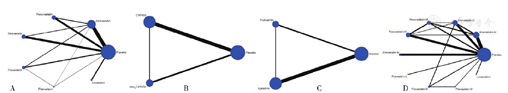

注:Placebo=安慰剂,Atorvastatin=阿托伐他汀,Rosuvastatin=瑞舒伐他汀,Simvastatin=辛伐他汀,Pravastatin=普伐他汀,Pitavastatin=匹伐他汀,Lovastatin=洛伐他汀,A为不同他汀类药物(简称他汀)之间的网络证据图,B为不同代谢途径他汀之间的网络证据图,C为不同亲脂性他汀之间的网络证据图,D为不同剂量他汀之间的网络证据图
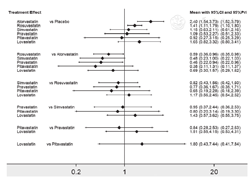
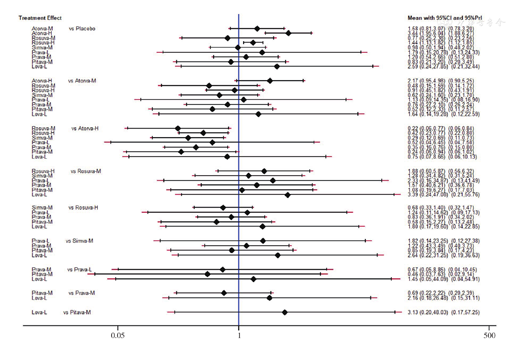
不一致性检验结果提示,不存在总体不一致性(χ2=2.82,P=0.727)和局部不一致性(见图5)。直接比较可见各研究间无统计学异质性(见图6)。网状Meta分析结果显示,阿托伐他汀肝功能异常发生率高于瑞舒伐他汀〔RR=1.70,95%CI(1.04,2.78)〕、普伐他汀〔RR=2.19,95%CI(1.06,4.52)〕,阿托伐他汀〔RR=2.40,95%CI(1.54,3.73)〕、瑞舒伐他汀〔RR=1.41,95%CI(1.11,1.79)〕肝功能异常发生率高于安慰剂,差异均有统计学意义(P<0.05,见图6)。SUCRA对不同他汀的排序结果为安慰剂(77.2%)>匹伐他汀(70.9%)>普伐他汀(64.1%)>辛伐他汀(60.7%)>瑞舒伐他汀(40.4%)>洛伐他汀(31.6%)>阿托伐他汀(5.1%)。
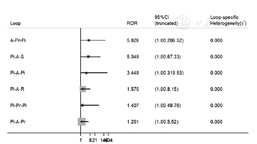

注:Pl=安慰剂,A=阿托伐他汀,R=瑞舒伐他汀,S=辛伐他汀,Pr=普伐他汀,Pi=匹伐他汀,L=洛伐他汀

按照他汀代谢途径对其进行分类[69]:通过细胞色素P450氧化酶(CYP450酶)进行代谢的他汀(CYP450):洛伐他汀[26,53,63,64]、辛伐他汀[24,28,29,50,52,54,55,66]、阿托伐他汀[23,25,30,32,34,37,39,40,44,45,46,47,49,51,56,60,61,62,65];不通过CYP450酶进行代谢的他汀(非CYP450):普伐他汀[27,30,48,51,67,68]、匹伐他汀[31,39]、瑞舒伐他汀[35,36,38,41,42,43,49,56,57,60]。不一致性检验结果提示,不存在总体不一致性(χ2=2.01,P=0.156)和局部不一致性〔ROR=1.96,95%CI(1.00,4.83)〕。直接比较可见各研究间无统计学异质性(见图7)。网状Meta分析结果显示,CYP450肝功能异常发生率高于安慰剂,差异有统计学意义〔RR=1.87,95%CI(1.34,2.63),P<0.05,见图7〕。SUCRA对不同他汀的排序结果为安慰剂(95.6%)>非CYP450(51.9%)>CYP450(2.6%)。
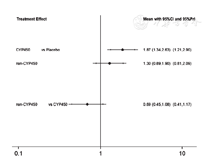

注:CYP450=通过细胞色素P450氧化酶进行代谢的他汀,non-CYP450=不通过细胞色素P450氧化酶进行代谢的他汀
按照他汀不同性质对其进行分类[69]:水溶性他汀:普伐他汀[27,30,33,48,51,67,68]、瑞舒伐他汀[35,36,38,41,42,43,49,56,57,60];脂溶性他汀:阿托伐他汀[23,25,30,32,34,37,40,44,45,46,47,49,51,56,60,61,62,65]、洛伐他汀[26,53,63,64]、辛伐他汀[24,28,29,50,52,54,55,66]、匹伐他汀[31,33]。不一致性检验结果提示,不存在总体不一致性(χ2=0.66,P=0.417)和局部不一致性〔ROR=1.41,95%CI(1.00,3.15)〕。直接比较可见各研究间无统计学异质性(见图8)。网状Meta分析结果显示,脂溶性他汀肝功能异常发生率高于安慰剂,差异有统计学意义〔RR=1.81,95%CI(1.30,2.51),P<0.05,见图8〕。SUCRA对不同他汀的排序结果为安慰剂(97.4%)>水溶性(48.7%)>脂溶性(3.9%)。
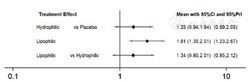

注:Hysrophilic=水溶性他汀,Lipophilic=脂溶性他汀
按照他汀治疗时LDL-C降低程度分为高强度他汀(LDL-C降低≥50%)、中强度他汀(LDL-C降低30%~49%)和低强度他汀(LDL-C降低<30%)[70]。高强度他汀:阿托伐他汀-H 40~80 mg[23,30,34,37,47,51,56,62,65]、瑞舒伐他汀-H 20~80 mg[35,38,41,42,43,56,57];中强度他汀:阿托伐他汀-M 10~20 mg[23,25,32,40,44,45,46,49,56,60,61,65]、瑞舒伐他汀-M 5~10 mg[36,42,49,56,57,60]、辛伐他汀-M 20~80 mg[24,29,50,52,66]、普伐他汀-M 40~80 mg[30,33,48,51,68]、匹伐他汀-M 1~4 mg[31,33];低强度他汀:普伐他汀-L 10 mg[27,67]、洛伐他汀-L 20 mg[26,64]。不一致性检验结果提示,不存在总体不一致性(χ2=2.13,P=0.995)和局部不一致性(见图9)。直接比较可见各研究间无统计学异质性(见图10)。阿托伐他汀-H肝功能异常发生率高于匹伐他汀-M〔RR=4.15,95%CI(1.07,16.08)〕、普伐他汀-M〔RR=2.87,95%CI(1.32,6.24)〕、辛伐他汀-M〔RR=3.50,95%CI(1.45,8.49)〕、瑞舒伐他汀-H〔RR=2.39,95%CI(1.30,4.39)〕、瑞舒伐他汀-M〔RR=4.49,95%CI(1.29,15.60)〕,阿托伐他汀-H〔RR=3.44,95%CI(1.95,6.04)〕、瑞舒伐他汀-H〔RR=1.44,95%CI(1.13,1.82)〕肝功能异常发生率高于安慰剂,差异均有统计学意义(P<0.05,见图10)。SUCRA对不同他汀的排序结果为瑞舒伐他汀-M(76.4%)>匹伐他汀-M(71.6%)>安慰剂(69.1%)>辛伐他汀-M(67.5%)>普伐他汀-M(55.1%)>普伐他汀-L(41.7%)>瑞舒伐他汀-H(40.9%)>阿托伐他汀-M(37.9%)>洛伐他汀-L(31%)>阿托伐他汀-H(8.8%)。
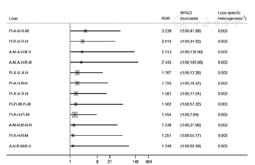

注:Pl=安慰剂,A-M=阿托伐他汀-M,A-H=阿托伐他汀-H,R-M=瑞舒伐他汀-M,R-H=瑞舒伐他汀-H,S-M=辛伐他汀-M,Pr-L=普伐他汀-L,Pr-M=普伐他汀-M,Pi-M=匹伐他汀-M,L-L=洛伐他汀-L

注:Atorva-M=阿托伐他汀-M,Atorva-H=阿托伐他汀-H,Rosuva-M=瑞舒伐他汀-M,Rosuva-H=瑞舒伐他汀-H,Simva-M=辛伐他汀-M,Prava-L=普伐他汀-L,Prava-M=普伐他汀-M,Pitava-M=匹伐他汀-M,Lova-L=洛伐他汀-L
他汀是目前应用最广泛的降脂药物,是治疗患者血脂异常、心脑血管疾病一级和二级预防最有效的方案。多项研究显示他汀每降低1 mmol/L低密度脂蛋白胆固醇(LDL-C),每年心脑血管事件发生率可降低20%,全因死亡率降低10%,卒中的相对风险降低近20%[10,15,71,72]。美国心脏病学会(ACC)/美国心脏学会(AHA)指南以及其他国际指南确立了他汀在降低动脉粥样硬化心脑血管病风险方面的首要地位[71,73]。但是他汀使用后出现的不良反应尤其是肝功能异常使其依从性受到一定程度限制。研究显示在心脑血管疾病一级和二级预防中停用他汀会明显增加心脑血管发病率及全因死亡率。HO等[74]对15 767名非致死性冠状动脉疾病患者随访4.1年,发现与坚持他汀治疗患者相比,不坚持治疗患者(服用他汀天数覆盖比例<80%)心血管住院率〔HR=1.35,95%CI(1.21,1.50)〕、心血管死亡率〔HR=1.62,95%CI(1.24,2.13)〕、冠状动脉血运重建〔HR=1.11,95%CI(1.01,1.22)〕、全因死亡率〔HR=1.85,95%CI(1.63,2.09)〕均明显升高。因此明确不同他汀对肝功能的影响很大程度影响其在临床的应用。但既往的研究中没有系统的多方面比较和评价不同他汀对肝功能的影响,因此本研究使用网状Meta分析,通过对RCTs进行直接和间接比较,旨在了解不同他汀对肝功能的影响,希望对临床使用他汀类药物提供帮助。
本研究通过对不同他汀行网状Meta分析显示,阿托伐他汀肝功能异常发生率高于瑞舒伐他汀、普伐他汀,阿托伐他汀、瑞舒伐他汀高于安慰剂,验证了NACI等[75]结果。他汀诱导的肝毒性可能源于广泛的肝脏代谢和亲脂性,剂量增高也可能导致肝损伤的风险增加[76],故本研究按照代谢途径、亲脂性、剂量对他汀分类进一步行网状Meta分析。
CYP450与CYP450酶抑制剂联用时,后者可抑制他汀的代谢,引起患者体内他汀浓度偏高,导致出现肝功能异常等不良反应,这一药物之间的相互作用已被充分认识[77,78],所以CYP450肝功能异常发生率较高。本研究结果证实了这一观点。因此在使用他汀时应慎重联用CYP450酶抑制剂。
脂溶性他汀主要通过被动扩散进入肝细胞,其亲脂性导致了有效的肝分流,同时也可导致其容易进入非肝细胞;水溶性他汀主要是由载体介导的广泛摄取进入肝细胞,故该类药物具有较高的肝选择性[69],因此脂溶性他汀出现不良反应尤其是肌肉疾病的概率高于水溶性他汀[15,79]。但是很少有研究探讨不同亲脂性他汀对肝功能的影响是否不同,本研究对此进行网状Meta分析,显示脂溶性他汀肝功能异常发生率较高。
对26个RCTs中17万名参与者的数据进行研究的Meta分析表明,使用强化他汀进一步降低LDL-C,可进一步减少心脑血管病的发生[4]。但是使用大剂量他汀强化治疗取得更好的治疗效果的同时肝功能障碍等不良反应也将显著增加[80],剂量-反应效应明显[81]。本研究对不同剂量的他汀进行比较,探讨不同剂量他汀对肝功能的影响,结果显示高强度他汀强化治疗(阿托伐他汀40~80 mg、瑞舒伐他汀20~80 mg)肝功能异常发生率高于低剂量他汀常规治疗(阿托伐他汀10~20 mg、瑞舒伐他汀5~10 mg),验证了以上观点。建议高心血管病风险患者根据2016年欧洲血脂异常治疗指南应用强化他汀治疗将LDL-C降至1.8 mmol/L以下,同时在用药初期对肝功能进行监测[82]。
为更好地实现心脑血管病一、二级预防,需要在取得他汀良好的治疗效果的同时尽可能避免出现肝功能异常,应结合患者种族、血脂情况、共病、目前使用药物等选择不同他汀治疗,尽可能选择水溶性他汀治疗,同时注意联合用药,避免使用CYP450酶抑制剂。在治疗前、治疗初3个月、增加剂量或更改用药时监测肝酶指标,不建议长期常规监测肝功能,但需要对有症状患者进行持续监测。对于ALT升高≥3ULN的患者,减少他汀的剂量或考虑替代药物治疗[15,83],尽量避免使用更易造成肝损害的他汀。本研究纳入文献较多且总体质量尚可,可以为临床选择他汀类药物及综合治疗提供帮助,但部分文献质量不高、一些干预措施纳入研究人数较少,仍需开展更多高质量的RCTs对本研究结果进行验证。
本文无利益冲突。



























