
Platelet-rich fibrin (PRF) is a second-generation platelet concentrate obtained from autologous blood. PRF is composed of abundant platelets, leucocytes, and a high concentration of various growth factors and fibrinogen. The composition and three-dimensional structure of PRF enable it to effectively make cells migrate and proliferate, playing an important role in tissue repair. Furthermore, the easy preparation and low cost of PRF make it a good treatment option. Numerous articles have been published about the application of PRF in clinical practice, however, the application of PRF in dermatology has not been comprehensively reviewed. The objective of this review article was to discuss various applications of PRF in dermatology, including healing chronic wounds, treating androgenic alopecia, skin rejuvenation, autologous fat transplantation, and treating vitiligo. PRF is a promising dermatologic treatment, but lacks a standardized protocol regarding its methods of attainment and use, which needs more investigations.

Copyright © 2022 Hospital for Skin Diseases (Institute of Dermatology), Chinese Academy of Medical Sciences, and Chinese Medical Association, published by Wolters Kluwer, Inc.
This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-No Derivatives License 4.0 (CCBY-NC-ND), where it is permissible to download and share the work provided it is properly cited. The work cannot be changed in any way or used commercially without permission from the journal.
Platelet-rich fibrin (PRF) is a second-generation platelet concentrate obtained from autologous blood. Compared with the first generation platelet concentrate, platelet-rich plasma (PRP), PRF is prepared in a natural manner without the addition of biochemical substances like anticoagulants or bovine thrombin to the blood sample before centrifugation1; this enables the use of a simpler production process while reducing the error rate in the preparation phase. PRF has a three-dimensional structure and contains a high concentration of growth factors including vascular endothelial growth factor (VEGF), transforming growth factor β1(TGF-β1), insulin-like growth factor (IGF), and cytokines such as interleukin-1β, interleukin-6β, and tumor necrosis factor-α.2 Moreover, scanning electron microscopic images have demonstrated that the PRF scaffold composed of compact fibrin strands and porosities essentially entraps platelets, enabling the steady release of growth factors for a sustained period of time.3 Given its simple preparation, autologous nature, low cost, and biological properties.4,5,6 In the field of dermatology, PRF has shown unique advantages in the treatment of various skin diseases and is expected to become a widely used and effective treatment.
With the searching terms of "PRF," "platelet-rich fibrin," "PRP," "platelet-rich plasma" and "dermatology," we searched in PubMed for the literature from January, 2006 to January, 2021. This review aimed to highlight the potential use and efficacy of PRF in various areas of dermatology such as the healing of chronic wounds, treatment of androgenic alopecia (AGA), skin rejuvenation, autologous fat transplantation, and vitiligo treatment.
Currently technique used to create platelet concentrates is a lack of uniformity to obtain and classify PRF. Dohan Ehrenfest et al.7 proposed a standard for the classification of platelet concentrates by leucocyte and fibrin concentrations pure PRP, leukocyte- and PRP, pure PRF, and leucocyte- and PRF. The most widely used type of PRF, Choukroun PRF, is a leucocyte- and PRF. This classification helps systematically evaluation of the research value in this field and the further development of this technology.
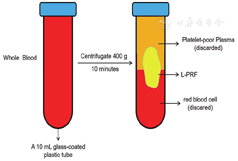
The method used to obtain PRF differs between studies, and we focused on the preparation of Choukroun PRF in this review article. The standard protocol for the preparation of Choukroun PRF is as follows.8 The first step is to collect peripheral blood without anticoagulant in a 10-mL glass-coated plastic tube. The blood is then divided into three layers by centrifugation at approximately 400g: red blood corpuscles at the bottom of the tube, platelet-poor plasma at the top, and an intermediate layer of PRF called the "buffy coat" The intermediate layer is the target (Fig. 1). PRF preparation is completely dependent on the centrifugation of blood and does not require any chemicals such as anticoagulants or thrombin. The coagulation cascade is initiated as soon as the blood contacts the tube. Circulating thrombin, which is transformed from prothrombin after initiation of the coagulation cascade, transforms the fibrinogen into fibrin during centrifugation. The fibrin in PRF forms a complex three-dimensional structure that effectively entraps a large number of platelets and leukocytes, allowing for a slow and long-lasting release of growth factors.1,8 If necessary, the PRF gel is pressed with sterile gauze, creating a stable membrane structure with certain elasticity, shape, and toughness.

New advanced versions of PRF have been created for clinical applications. One of the main limitations of PRF is its fibrin scaffold consistency, which makes it difficult to combine with bone biomaterials. To overcome this shortcoming, an injectable formulation of PRF has been developed without any anticoagulants. Injectable PRF is formulated at lower speeds (60 g) for 3 minutes.9 The use of horizontal centrifugation to produce PRF may result in a fourfold increase in the number of immune cells in PRF.10 As leukocytes are a crucial part of the immune system, horizontally centrifuged PRF may show greater antibacterial effects than typical PRF. In addition, horizontal centrifugation of PRF results in better cell layer separation. A similar modified PRF obtained by centrifugation at 1,300 rpm for 8 minutes, referred to as advanced PRF,11 also contains a greater number of leukocytes than typical PRF. Despite the abovementioned efforts made by innovative researchers, there is still a need for further studies to provide standardized PRF production protocols.
The composition and structure of PRF are the basis of its function. The main components of PRF are abundant platelets, leucocytes, a high concentration of various growth factors, and fibrinogen that is converted into fibrin. In addition to the main function of platelets in the blood circulation, they also help maintain the hemostasis and blood flow in blood vessels.12 Platelets are activated during centrifugation and release a granules that carry various growth factors and bioactive proteins. Thus, PRF contains high concentrations of growth factors, including platelet-derived growth factor (PDGF), VEGF, TGF-β1, and IGF. These growth factors activate downstream signaling pathways by binding to the corresponding receptors on cell membranes, and transmit signals into the nucleus to take part in various activities such as inflammation, collagen synthesis, tissue granulation, and angiogenesis. PDGF is considered the critical trigger of the tissue repair process, and contributes to the proliferation of fibroblasts and other mesenchymal cells.13 VEGF stimulates the synthesis of blood vessels and the basal lamina and enhances the microvascular permeability.14 TGF-β1 regulates cell motility, differentiation, and proliferation, and plays an important role in physiological repair by promoting collagen production and accumulation.15,16 Characterization of the biological properties of different platelet concentrates in vitro revealed that PRF contains greater amounts of released TGF-β1 and results a prolonged release of growth factors and stronger induction of cell migration.17 IGF promotes mitosis and the proliferation of fibroblasts.18 Notably, the growth factors in PRF are not equally distributed and form a concentration gradient from high at the lower part to low at the upper part.19 This feature must be considered in the application of PRF.
The most notable soluble constituent in PRF is fibrinogen, a clotting protein. During centrifugation, fibrinogen naturally and slowly transforms into insoluble fibrin, forming a three-dimensional network. The fibrin network is crucial in constituting the elements responsible for the therapeutic potential of PRF.20 This network helps spread nutrients and oxygen to the surrounding areas, and acts as a scaffold for cell attachment, migration, and differentiation. As mentioned previously, this three-dimensional structure effectively entraps platelets and various circulating molecules. Studies have shown that the structure of PRF is beneficial for the synergistic action between growth factors and prolonged function.21 It has also been suggested that PRF prevents growth factors from undergoing proteolysis, which helps prolong the secretion time of growth factors.22 In conclusion, growth factors and the three-dimensional fibrin network of PRF create a controlled release system to maintain biological activity.
The specific parameters of the centrifugation protocols used in all cited studies are provided in brackets (except the studies didn't provide).
Chronic wounds, especially non-healing chronic wounds, have brought a huge physiological, psychological, and economic burden to patients and society. The traditional clinical treatment methods (dressing, debridement, and skin flap transplantation) are not effective, driving dermatologists to find better treatment options. In the field of oral surgery, PRF leads to more effective cell migration and proliferation and thus improved wound healing.23 Furthermore, Crisci et al.23 successfully treated diabetic ulcers using PRF (centrifugation protocol: 30 seconds acceleration, 2 minutes at 2,700 rpm, 4 minutes at 2,400 rpm, 3 minutes at 3,000 rpm, and 36 seconds deceleration and stopping), with the skin lesions healing without infection in all patients. A randomized, triple-blind study showed that PRF (centrifugation protocol: 10 minutes at 3,000 rpm) significantly increases the time and speed of wound healing compared with conventional treatment, and also reduces the pain severity.24 Although pain perception is subjective, other studies have reported a significant decrease in pain with PRF application.23,24,25 These findings show that PRF has good potential as a new method with which to treat chronic wounds.
Another crucial factor affecting wound healing is the presence of leukocytes. Infection is one of the most common causes of non-healing chronic wounds. The numerous leukocytes trapped in PRF release inflammatory factors and anti-inflammatory factors after being activated during centrifugation. These factors bind to the fibrin network and reduce the immune response, showing the ability to regulate inflammation and resist infection.8 In addition, PRF has long-term antibacterial effects against Fusobacterium nucleatum and Staphylococcus aureus. A modified PRF preparation (centrifugation protocol: 12 minutes at 2,700 rpm) may be used to reduce the risk of infection in addition to the other beneficial properties of PRF.26
Liquid PRF can also be used as an advanced local delivery system for biomolecules,27 and thus may be used as a drug carrier to promote tissue regeneration. This may become a popular research topic in the future. Furthermore, with the development of biomaterials, the properties of PRF may be changed by combining it with various materials for use in treating different types of wounds, improving the effectiveness of PRF.
Regenerative therapies have become a growing demand in the field of facial esthetics. The changes associated with aging can be reduced by increasing the collagen and hyaluronic acid content. Blood products contain a large number of growth factors that stimulate fibroblast formation and subsequently increase the collagen and hyaluronic acid content of skin. Therefore, PRP has particularly attracted the attention of dermatologists in the field of skin rejuvenation. People who received injections of PRP (centrifugation protocol: 5 minutes at 3,000 rpm) developed collagen thickening due to upregulated levels of metalloproteinases 1 and 2,28 whose main function is to degrade collagen and elastin. Furthermore, fluid PRF (centrifugation protocol: 3 minutes at 60 g) stimulates greater dermal skin fibroblast cell migration and proliferation and collagen synthesis compared with PRP (centrifugation protocol: 5 minutes at 900 g, then 15 minutes at 2,000 g).29 As mentioned above, PRF has a natural three-dimensional structure that acts as a scaffold and facilitates prolonged activities of growth factors. Karimi et al.30 found that injecting PRF alone as a natural, autologous filler results in volume restoration of hollowing tear troughs, homogenization of pigmentation irregularities with repeat treatments, and improvements in fine lines; furthermore, a combination of PRF and hyaluronic acid filler synergistically improves moisture retention and provides a scaffold for collagen growth. Compared with traditional skin rejuvenation treatments such as chemical peeling, botulinum toxin injection, and local laser therapy, PRF is much safer due to its fully autologous nature and is a cost-effective choice. Because of its size and shape, PRF is easy to combine with many other techniques, especially in surgery and cosmetic dermatology. The role of PRF in skin rejuvenation is an exciting prospect and may ultimately lead to superior therapies in the future. Nevertheless, there is a definite need for more extensive independent studies and double-blind clinical trials of PRF in accordance with the requirements of evidence-based medicine, although the use of PRP in this area has been well documented.
AGA is one of the most common problems in both sexes encountered in clinical dermatology. Drugs (topical minoxidil and oral finasteride) and low level 655-nm laser devices are widely employed to treat AGA. Another treatment option for AGA is injections of PRP (centrifugation protocol: 10 minutes at 1,200 rpm), which increases hair density and hair follicular bulge cells.31 A high-quality systematic review comparing the efficacy of various non-surgical treatments for AGA showed that although PRP is not the most effective therapy, it has the highest degree of safety and shows great potential.32 There are many proposed methods of using PRP in the management of AGA, including interfollicular PRP injection and PRP mesotherapy. PRF is an advanced version of PRP that has been used by some researchers to treat AGA. Schiavone et al.33 used injectable PRF (centrifugation protocol: 5 minutes at 1,500 rpm) to produce positive clinical results in patients with AGA. They observed some degree of clinical improvement in patients with all levels of disease severity at baseline and in both sexes. In particular, patients with a greater degree of disease severity at baseline tended to achieve a larger improvement after treatment.33 However, compared with PRP, the application of PRF in the management of hair loss is still in the very beginning of the exploratory stage and requires evaluation in high-quality randomized controlled trials. PRF can be used as an independent therapy for AGA. Some dermatologists have also applied some innovative ideas such as using PRP as an auxiliary treatment in combination with follicular unit extraction surgery or drugs. However, it remains unclarified how, when, and for how long to combine these treatment methods. There is still a long way to go to progress from ideas to practical guidelines in dermatology. In addition, apart from AGA, the etiologies of hair loss are very diverse and there is little published information about the application of PRF in the management of other kinds of hair loss; thus, there is a need to expand the scope of PRF application.
Various methods are used to improve the survival rate of transplanted fat and it is important to not only promote the establishment of blood supply in the recipient area, but to also reduce the decomposition of fat cells and promote the proliferation of fibrous tissue in the body. PRF can perform these functions. It has been proved that PRF (centrifugation protocol: 10 minutes at 3,000 rpm) postpones or inhibits the apoptosis of adipocytes, promotes angiogenesis and adipogenesis, and regulates collagen production to improve fat graft survival.34 This suggests that PRF application is a promising method with which to assist autologous fat transplantation. So far, many studies have evaluated the use of PRP in fat transplantation, but few have evaluated the use of PRF. A double-blinded prospective clinical trial in which 25 patients underwent bilateral facial lipostructure surgery in the cheek and cheekbone areas by combining fat with PRP or PRF revealed a slight esthetic asymmetry with greater average resorption at 1 year postoperatively on the side treated with PRP (centrifugation protocol: 10 minutes at 3,000 rpm) and fat compared with the side treated with PRF and fat.35 Therefore, the combination of fat and PRF is more effective than fat and PRP in facial lipostructure surgery. Furthermore, fat acquisition, processing, and grafting techniques are considered to be main factors affecting the volume retention rate of fat grafting. PRF (centrifugation protocol: 10 minutes at 3,000 rpm) has different effects on macrofat versus shuffling fat grafting. A study demonstrated that the group of patients who received shuffling fat containing PRF showed superior microcosmic evaluation compared with the group who received macrofat containing PRF.36 In summary, combining PRF with autologous fat transplantation is an ideal and promising surgical method in surgical and esthetic dermatology that is worthy of clinical promotion. However, there are still some problems to be resolved. For example, the ratio of PRF to fat grafts varies between studies, and further investigation is needed to determine the optimal ratio of PRF to fat grafts.
In the past few decades, the potential use of PRP in the treatment of vitiligo has attracted an increasing amount of attention. The effectiveness of PRP in the treatment of vitiligo may be related to the high concentrations of growth factors, which may help stimulate the proliferation of melanocytes and result in repigmentation. As the PRP preparation process does not involve the addition of anticoagulant in the blood collection tube, the blood should be centrifugated as soon as possible so that natural coagulation does not occur and result in the fibrin being polymerized in the form of dispersion. In addition, to ensure the high quality of PRF, it should be used within 1 hour after preparation because research has shown that the concentrations of EGF, VEGF, TGF-β1, PDGF-AB, and other growth factors in PRF gradually increase within 1 hour after preparation and are rapidly released within 2-5 hours37 Kadry et al.38 studied the effect of PRP (centrifugation protocol: 5 minutes at 1,500 rpm), fractional CO2 laser therapy, and a combination of both PRP and fractional CO2 laser therapy in 30 patients with vitiligo. The combination of both fractional CO2 laser therapy and PRP achieved the best results, followed by PRP alone, and then fractional CO2 laser therapy alone. Another more recent study evaluated nearly all vitiligo treatment modalities by comparing the safety and efficacy of PRP (centrifugation protocol: 10 minutes at 1,000 rpm, then 10 minutes at 2,000 rpm), fractional CO2 laser therapy, combined PRP and fractional CO2 laser therapy, combined NB-UVB therapy and fractional CO2 laser therapy, and combined PRP, fractional CO2 laser therapy, and NB-UVB39; of these treatments, the triple therapy achieved the most significant improvement in vitiligo symptoms. These findings all indicate the usefulness of PRP as an adjunctive treatment for vitiligo. Although PRF shows great advantages over PRP in many aspects, to our knowledge, no study has evaluated the application of PRF in treating vitiligo.
PRF is widely used in dermatology. In addition to the abovementioned applications, PRF has been applied in other aspects of dermatology, showing the advantages of blood products compared with conventional treatment. However, we have not discussed these applications in detail because of a lack of reliable evidence. For example, for striae distensae, a challenging cosmetic problem commonly encountered by dermatologists, a short treatment duration of PRP (centrifugation protocol: 7 minutes at 1,419 g, then 5 minutes at 2,522 g) alone is more effective than microdermabrasion alone, and the combination of both PRP and microdermabrasion is much more effective.40 PRP (centrifugation protocol: 10 minutes at 160 g, then 10 minutes at 400 g) has also been used to enhance the therapeutic efficacy of laser treatment for acne scars; the PRP treatment improved the clinical efficacy with decreased severity of adverse effects such as swelling, oozing, and erythema.41 A pilot study in which intradermal injection of autologous PRP (centrifugation protocol: no specific parameters) was used to treat vulvar lichen sclerosus showed that PRP significantly decreases the histopathologic inflammation in women with vulvar lichen sclerosus, without the adverse effects related to topical or systemic immunomodulators.42 These studies suggest that PRF has a wide application in various aspects of dermatology, although PRP is much more commonly used at present.
Although various studies have attempted to identify the optimal protocol with which to obtain the highest concentration of platelets and growth factors, the creation of a standardized protocol for PRF preparation is complex. The centrifuge characteristics and centrifugation protocols have a significant impact on the cells, growth factors, and fibrin structure of PRF,43 and the variety of centrifuges and units of measure used in different studies hinders the creation of a standardized protocol and affects the repeatability of results.44 Choukroun and Ghanaati proposed the concept of low-speed centrifugation, which has been proven to improve the effectiveness of PRF.45 However, more standardized studies are necessary to determine the optimal effect.
A standardized protocol with which to obtain high-quality PRF is still under investigation and the protocol may differ in various specific clinical scenarios. The required amount of PRF is small and the storage is inconvenient; therefore, PRF cannot be produced in large quantities or widely used in clinical practice. PRF has many reported benefits, while there have been no reports of major complications and disadvantages. The difficulty in demonstrating conclusive evidence of the beneficial effects of PRF is due to a lack of randomized controlled trials in humans and a lack of experimental research. Additionally, the newest generation of platelet concentrates, concentrated growth factor (CGF), has a denser and more stable fibrin network than PRF.46 One study proved that the concentrations of growth factors were greater in CGF than in PRF, regardless of the amount and concentration.47 This suggests that CGF may have greater potential than PRF in clinical application.
The tube material used in the PRF preparation process may have a crucial effect due to the unavoidable contact with the silica in glass tubes. Although the silicon dioxide particles are dense enough to sediment with the red blood cells, they are small enough to remain suspended in the fibrin and buffy coat. To avoid the possible health hazard of glass-evacuated collection tubes with silica activators, some recent studies have used titanium-prepared PRF by replacing the traditional glass tube used for venous blood centrifugation; however, the high cost of titanium-prepared PRF limits the widespread use of this new option.
Although the beneficial or detrimental effects of leukocytes has always been controversial, their antimicrobial effects are beneficial. Furthermore, it has been discovered that the maximal amount of both leukocytes and platelets, as well as the maximal leukocyte/platelet ratio, in PRF are obtained by using the manufacturer’s protocol with 400 g.48
Numerous studies published in peer-reviewed journals show that PRF has a wide range of dermatological applications ranging from healing chronic wounds, skin rejuvenation, AGA treatment, autologous fat transplantation, and vitiligo treatment to hair restoration and even the treatment of relatively uncommon diseases like vulvar lichen sclerosus. PRF is being used in many new areas of dermatology, either alone or in combination with traditional therapies.
PRF as an autologous blood derivative with the features of simple production, a high degree of safety, and low price, and is becoming widely used in clinical practice. The main complications and disadvantages of PRF have not yet been reported. The usage of PRF in healing chronic wounds, skin rejuvenation, AGA treatment, autologous fat transplantation, and vitiligo treatment is relatively common. However, further studies are needed to expand the scope of PRF application in other specific areas of clinical dermatology. There also remain some problems and limitations of PRF treatment, mainly regarding the lack of a standardized protocol and lack of large-scale studies. With the continuous development of biological and medical science, the application of PRF in dermatology will become more standardized and systematic, and a deeper understanding of the mechanism of PRF will be obtained.
The authors reported no conflicts of interest.






























